Abstract
There is growing evidence that antitumor treatment contributes to better seizure control in low-grade glioma patients. We performed a systematic review of the current literature on seizure outcome after radiotherapy and chemotherapy and evaluated the association between seizure outcome and radiological response. Twenty-four studies were available, of which 10 described seizure outcome after radiotherapy and 14 after chemotherapy. All studies demonstrated improvements in seizure outcome after antitumor treatment. Eight studies reporting on imaging response in relation to seizure outcome showed a seizure reduction in a substantial part of patients with stable disease on MRI. Seizure reduction may therefore be the only noticeable effect of antitumor treatment. Our findings demonstrate the clinical relevance of monitoring seizure outcome after radiotherapy and chemotherapy, as well as the potential role of seizure reduction as a complementary marker of tumor response in low-grade glioma patients.
Keywords: chemotherapy, epilepsy, glioma, radiotherapy, review
Seizures affect 30%–90% of patients with a glioma and are particularly prevalent in patients with low-grade glioma (LGG).1–5 Despite antiepileptic drug (AED) treatment, 15%–35% of patients still experience seizures.6,7 Uncontrolled, seizures may result in high morbidity and negatively impact quality of life.8,9 Therefore, achieving seizure control is an important challenge in the clinical management of LGG.10
There is growing evidence that the antitumor treatment itself may lead to better seizure control in LGG patients. Several studies have described a reduction in seizure frequency or seizure freedom after surgery, radiotherapy, and chemotherapy, which means a direct clinical benefit for the patient.7,11–15 Furthermore, the effect of antitumor treatment on seizure frequency could be of value in the assessment of tumor response. Currently, tumor response assessment according to the Response Assessment in Neuro-Oncology (RANO) criteria is largely based on MRI.16,17 However, radiological assessment can be rather difficult, particularly after radiotherapy and chemotherapy. In a substantial part of LGG patients, a clinical improvement after nonsurgical treatment is not accompanied by an objective radiological response.18–20 Thus, in some patients the radiological response does not fully reflect the actual benefit of the treatment. It is therefore of major interest to determine possible complementary outcome measures after antitumor treatment.
With this systematic review we aim to increase the knowledge of the course of epilepsy after antitumor treatment, by giving a comprehensive overview of (i) the existing literature on seizure outcome after radiotherapy and chemotherapy in patients with LGG and (ii) the association between seizure outcome and radiological response after antitumor treatment.
Materials and Methods
Search Strategy and Selection Criteria
We performed a literature search using the electronic resources PubMed and Embase until August 2014. The complete search strategy is outlined in Supplementary Table S1. The search included a combination of search terms related to “glioma,” “radiotherapy or chemotherapy,” and “seizure outcome,” limited to English language and human studies.
We followed the guidelines of PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses).21 Two authors (J.A.F.K. and M.K.) determined whether the articles were eligible for inclusion and served as reviewers of the full texts of all selected articles. Inclusion criteria were: (i) adult patients with histologically proven World Health Organization (WHO) grade II glioma, (ii) received radiotherapy (focal fractionated irradiation, stereotactic radiotherapy, or brachytherapy) or chemotherapy, (iii) a sample size ≥5. Initial exclusion criteria were: (i) reviews, (ii) abstracts not published as full papers. Additionally, we applied the following exclusion criteria to the remaining articles: (i) no seizure outcome reported after antitumor treatment (radiotherapy or chemotherapy) (n = 306), (ii) only acute symptomatic seizures described (n = 19), (iii) studies with children (n = 2), and (iv) studies with HGG patients only (n = 1). We searched the reference lists of the selected full text articles to identify additional studies. From the selected articles, we extracted the following data: study design, sample size, demographic and clinical characteristics of the study population, type of radiotherapy or chemotherapy, additional antitumor treatment, time of seizure assessment, and seizure outcome (seizure frequency, seizure reduction, Engel class, and/or seizure freedom). If available, we reported the radiological response in relation to seizure outcome.
Results
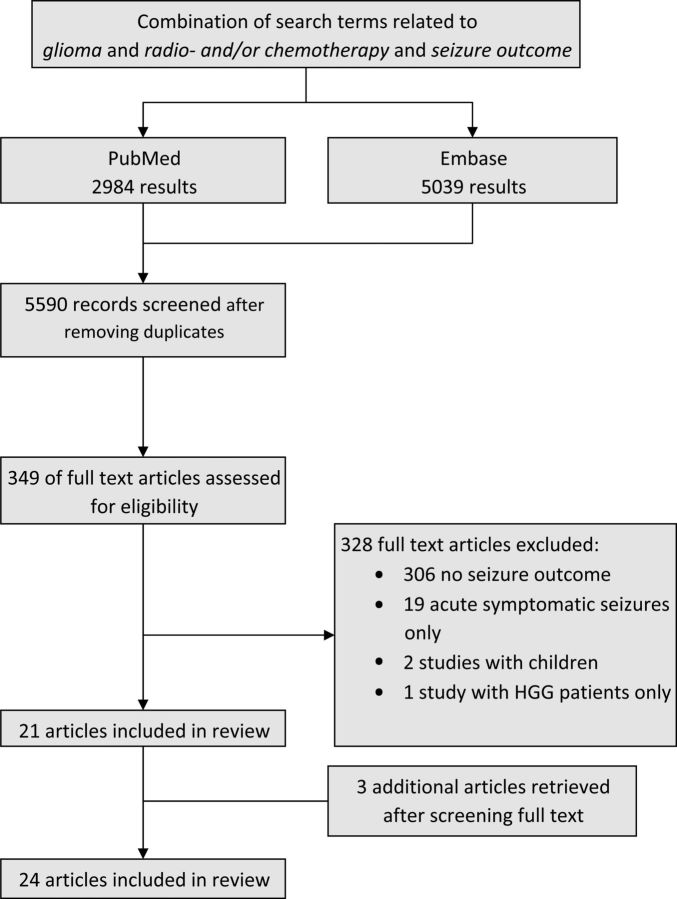
The search yielded 5590 unique records, of which we assessed 349 full text articles for further eligibility (Fig. 1). After exclusions, eventually we included 21 articles and found 3 additional studies by screening the reference lists of the remaining full text articles.22–24 The available 24 studies were categorized into the following 3 topics: (i) seizure outcome after radiotherapy (10 studies), (ii) seizure outcome after chemotherapy (14 studies), and (iii) seizure outcome in relation to radiological response (8 studies). All eligible studies are discussed in the context of these 3 topics in the following sections.
Fig. 1.
Identification of articles.
Seizure Outcome After Radiotherapy
The key findings of all 10 studies that described seizure outcome after radiotherapy are outlined in Table 1.13,25–33 We found 8 patient series13,25–27,29–31,33 and 1 randomized controlled trial.32 The design of one study was unclear.28 However, in all 10 studies, seizure data were collected retrospectively. Patients had received focal fractionated irradiation (4 studies),13,26,27,32 brachytherapy (3 studies),28,31,33 or stereotactic radiotherapy (3 studies).25,29,30 Five studies included patients with LGG only,25–27,33 3 studies included patients with WHO grade II or grade III glioma,13,28,32 and 2 studies included mainly patients with LGGs but also a small group with other, nonglial brain tumors.29,30 In 5 studies, radiation was the first antitumor treatment,26–28,31,33 in 4 studies a proportion of the patient group had previously undergone resection,13,25,30,32 and in 1 other study previous antitumor treatment was not specified.29 The number of patients in whom seizure outcome was assessed ranged considerably, from 5 to 173 cases. Four studies included patients both with and without a history of epilepsy,25,26,31,32 2 other studies included only patients with a history of epilepsy,30,33 and 4 other studies included only patients with medically intractable epilepsy.13,27–29
Table 1.
Summary of seizure outcome after radiotherapy
| Article | Study Design | Population (baseline) | Treatment | Additional Treatment, n | Time of Seizure Assessment | Seizure Outcome (% total population) |
|---|---|---|---|---|---|---|
| Rossi et al, 198528 | N/S | Malignant brain tumor and medically intractable epilepsy 15: PA 1/A 9/OA 2/O 2/AA 2 | 125I brachytherapy | None | N/S | Reduction in seizure frequency: 15/15 (100%) Seizure freedom: 8/15 (53%) |
| Rogers et al, 199327 | Retrospective | LGG and medically intractable epilepsy 5 | Focal fractionated irradiation | None | N/S | >90% Seizure reduction: 3/5 (60%) 75%–90% Seizure reduction: 1/5 (20%) Seizure freedom: 1/5 (20%) |
| Scerrati et al, 199431 | Prospective | Grade I/II glioma 36 (34 with history of epilepsy): PA 2/A 23/O 11 |
Interstitial brachytherapy | None | 12 m after therapy | Seizure freedom: 19/34 (56%) |
| Warnke et al, 199733 | Retrospective | WHO grade II astrocytoma with a history of epilepsy 80; 22 (28%) with seizure freedom | Interstitial radiosurgery with 125I temporary implants | None | 3 m after therapy 6 m after therapy |
Increase in percentage of patients with: Seizure freedom (from 28% to 68%) Seizure freedom (from 28% to 80%) |
| Schröttner et al, 199829 | Retrospective | Medically intractable tumor epilepsy 26: 17 LGG/9 other | Gamma knife radiosurgery | N/S | N/S | Engel class I/II: 13/24 (54%) Engel class III: 4/24 (17%) |
| Schröttner et al, 200230 | Retrospective | Mesiotemporal tumor epilepsy 19: LGG 15/GG 3/cavernoma 1 | Gamma knife radiosurgery | Resection 10/19 (53%) | N/S | Engel class I and II (significant reduction): 11/19 (58%) Engel class III (worthwhile improvement): 7/19 (37%) |
| Plathow et al, 200325 | Retrospective | WHO grade II astrocytoma 143; 52 with general seizures and 49 with focal seizures | Fractionated stereotactic irradiation | Complete resection: 26/143(18%) Subtotal resection: 40/143 (28%) |
6w after therapy | Decrease in percentage of patients with: Generalized seizures (from 36% to 7%) Focal seizures (34% to 17%) |
| Shankar and Rajshekhar, 200326 | Both retrospective and prospective | Insular low-grade astrocytoma 30 (26 presented with epilepsy) | Radiotherapy (unspecified) | None | N/S | Engel class I: 21/30 (70%) Engel class II: 4/30 (13%) Engel class III: 1/30 (3%) |
| van den Bent et al, 200532 | Prospective | (1) Glioma 157: A 78/OA 25/O 19/PA 2/GrIII 20/other 13 (2) glioma 154: A 80/OA 15/O 23/PA 2/GrIII 28/other 6 (no significant difference in number of patients with seizure control between groups 1 and 2) |
Focal fractionated irradiation (late) Focal fractionated irradiation (early) |
Resection: 93/157 (59%) Resection: 99/154 (64%) |
12 m after therapy 12 m after therapy |
Seizure freedom: 29/71 (59%) Seizure freedom: 26/102 (75%) |
| Ruda et al, 201313 | Retrospective | Glioma and medically intractable epilepsy 43: AII-III 28/OAII-III 5/OII-III 10 |
Focal fractionated irradiation | Resection: 29/43 (67%) | 3 m after therapy 12 m after therapy |
≥50% Seizure reduction: 31/43 (72%) Seizure freedom: 15/43 (35%) ≥50% Seizure reduction: 26/34 (77%) Seizure freedom: 13/34 (38%) |
Abbreviations: PA, pilocytic astrocytoma; A, astrocytoma grade II; OA, oligoastrocytoma grade II; O, oligodendroglioma grade II; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; GG, ganglioglioma; N/S, not specified; m, months.
All studies reported an improved seizure outcome after radiotherapy; however, different seizure outcome measures were used, such as the percentage of patients showing a reduction in seizure frequency, seizure freedom, and improved Engel class. A reduction in seizure frequency, ranging from ≥50% to >75%, was reported in 3 studies in 72%–100% of patients.13,27,28 One study that included WHO grades II–III glioma patients on a stable AED dose treated with focal fractionated irradiation reported a ≥50% seizure reduction in 72% after 3 months, and in 77% at 12 months after radiotherapy. Seizure reduction appeared to be more common in patients with a long history of seizures before the start of radiotherapy.13 One smaller series evaluating seizure outcome after focal radiotherapy reported a >75% seizure reduction in 4/5 patients.27 In a series of 15 patients receiving brachytherapy, all patients showed a reduction in seizure frequency, although the extent of the reduction was not reported.28 In a series of 26 patients treated with gamma knife radiosurgery in different doses, 66% of patients treated with a high dose reported Engel class I or II compared with 42% in the low-dose group.29
Seizure freedom was reported in 9 articles, ranging from 20% after focal radiotherapy27 to 80% at 6 months after brachytherapy.28 In one study that compared early versus late radiotherapy in patients with LGG, 59% of patients were seizure free 12 months after treatment in the late radiotherapy group, compared with 75% of patients in the early radiotherapy group.32 Before the start of radiotherapy, there were no differences in the number of patients with controlled seizures between the 2 groups.32 In a large cohort of 143 patients with LGG treated with stereotactic radiotherapy evaluating seizure outcome 6 weeks posttreatment, the percentage of patients reporting seizures decreased from 70% to 24%.25 Two other studies on brachytherapy reported seizure freedom after 6 months in 80% of patients initially suffering from seizures,33 and after 12 months in 56% of patients.31
Seizure Outcome After Chemotherapy
In total, 14 studies described seizure outcome after chemotherapy.12,22–24,34–43 The key findings of all studies are outlined in Table 2. We found no randomized controlled trials. Seven studies had a prospective noncontrolled design,22,35,36,38,41,42 and the 7 other studies were retrospective,12,23,34,37,39,40,43 of which 1 study had included a control group of patients with LGG under observation.12 All studies reported patients with diffuse WHO grade II glioma including astrocytoma, oligoastrocytoma, and/or oligodendroglioma. Patients had been treated with either temozolomide (TMZ; 8 studies)12,23,24,35,36,38,40,41 or procarbazine-lomustine-vincristine (PCV; 4 studies)22,34,39,42 or had received different types of chemotherapy (TMZ, PCV, fotemustine, cisplatin, or etoposide; 2 studies).37,43 In all studies except one,37 patients had received other antitumor treatment before chemotherapy was administered: in 9 studies part of the patients had undergone surgery alone,12,22–24,35,36,38,39,43 in 1 study radiotherapy alone,34 and in 3 studies surgery and/or radiotherapy.40–42 The number of patients in whom seizure outcome was analyzed ranged from 9 to 149 subjects. The studies included patients with a history of epilepsy (8 studies),12,22,34–36,39,42,43 uncontrolled seizures despite AED treatment (4 studies),24,37,40,41 or an unknown seizure status before the start of chemotherapy (2 studies).23,38
Table 2.
Summary of seizure outcome after chemotherapy
| Article | Study Design | Population (baseline) | Treatment | Additional Treatment, n | Time of Seizure Assessment | Seizure Outcome (% total population) |
|---|---|---|---|---|---|---|
| Mason et al, 199622 | Prospective | Newly diagnosed and recurrent LGG 9 (6 with epilepsy) | PCV | Resection: 5/9 (56%) | N/S | Improved seizure control: 6/6 (100%) |
| Soffietti et al, 199842 | Prospective | Progressive LGG 26 (23 with epilepsy and/or neurological deficits): OA 9/O 17 |
PCV | Resection: 23/26 (88%) Radiotherapy: 11/26 (42%) |
At 4-wk intervals during chemotherapy | Improved seizure control: 7/23 (30%) Seizure freedom: 3/23 (13%) |
| Brada et al, 200336 | Prospective | Stable or progressive LGG 30 (27 with history of epilepsy): A 17/OA 2/O 11 |
TMZ | Resection: 12/30 (40%) | During chemotherapy (24 patients completed 12 cycles) | Improvement in seizure frequency: 14/27 (52%) |
| Pace et al, 200341 | Prospective | Progressive LGG 43 (31 with uncontrolled epilepsy): A 29/OA 4/O 10 |
TMZ | Resection: 32/43 (74%) Radiotherapy: 30 (70%) PCV: 16 (37%) |
Every 3 TMZ cycles | ≥50% Seizure reduction: 15/31 (48%) Seizure freedom: 4/31 (13%) (In patients with previously uncontrolled epilepsy) |
| Hoang-Xuan et al, 200438 | Prospective | Progressive LGG 60: O 49/OA 11 (seizure status unknown) |
TMZ | Resection: 27/60 (45%) | N/S | Neurological improvement (eg, a reduction in seizure frequency): 30/59 (51%) |
| Biemond-ter Stege et al, 200534 | Retrospective | O and OA, newly diagnosed or recurrent 21 (20 with epilepsy) | PCV | Radiotherapy: 5/21 (24%) | N/S | Improved seizure control in most of 16 patients showing radiological response to treatment |
| Frenay et al, 200537 | Retrospective | A 10 (9 with epilepsy; 8 with pharmacoresistant epilepsy) | PCV/F-C-E | None | After 2nd course | Seizure reduction: 100% Seizure freedom: 60% |
| Kaloshi et al, 200723 | Retrospective | Progressive LGG 149: O 105/A-OA 44 (seizure status unknown) |
TMZ | Resection: 68/149 (46%) | Unknown; general follow-up: 30.4 m (range 2–70 m) | ≥50% Seizure reduction: 87/149 (58%) |
| Lebrun et al, 200739 | Retrospective | O 33 (24 with epilepsy at tumor presentation) | PCV | Resection: 7/33 (21%) | During chemotherapy (mean of 5 courses) | Seizure reduction: 53% Seizure freedom: 31% |
| Tosoni et al, 200824 | Prospective | Recurrent or progressive LGG 30 (13 with intractable seizures): A 9/OA 3/O 18 |
TMZ | Resection: 20/30 (67%) | After beginning of TMZ treatment | Seizure frequency reduction: 8/13 (62%) |
| Taillandier et al, 200943 | Retrospective | Insular LGG 21 (20 with epilepsy): A 3/OA 1/O 15/LGG N/S 2 | TMZ/PCV/F | Resection: 5/21 (24%) | N/S | Improved Engel class: 16/20 (80%) Seizure freedom: 8/20 (40%) |
| Blonski et al, 201235 | Prospective | Unresectable LGG and seizure at tumor presentation 10: A 2/OA 2/O 6 |
Neoadjuvant TMZ | Resection 3/10 (30%) | N/S | Seizure frequency reduction: 9/10 (90%) Seizure freedom: 5/10 (50%) |
| Sherman et al, 201112 | Retrospective | (1) LGG and seizure at tumor presentation 39 (12 patients seizure free with AED): A 3/OA 11/O 22/LGG N/S 3 (2) LGG controls with seizure at presentation 30 (14 patients seizure free with AED) |
TMZ Observation |
Resection: 24/39 (62%) Resection: 13/30 (43%) |
After median of 7 TMZ cycles N/S |
≥50% Seizure reduction: 23/39 (59%) ≥50% Seizure reduction without AED changes: 7/39 (18%) ≥50% Seizure reduction: 4/30 (13%) ≥50% Seizure reduction without AED changes: 0 |
| Koekkoek et al, 201440 | Retrospective | 66 LGG patients with uncontrolled epilepsy: A 43/OA 9/O 14 |
TMZ | Resection: 37/66 (56%) Radiotherapy: 46/66 (70%) |
After 6 m | ≥50% Seizure reduction without AED changes: 29/66 (44%) Seizure freedom: 27/66 (41%) |
Abbreviations: A, astrocytoma grade II; OA, oligoastrocytoma grade II; O, oligodendroglioma grade II; PCV, procarbazine, lomustine, and vincristine; F, fotemustine; C, cisplatin; E, etoposide; N/S, not specified; m, months.
A reduction in seizure frequency was described in 10 of 14 studies and varied from 48% to 100%.12,23,24,35–41 Four of these 10 studies had defined seizure reduction as a ≥50% reduction in seizure frequency, and the 6 other studies did not specify their definition of a seizure reduction. In 3 studies describing seizure reduction, the timing of seizure assessment was not specified,23,35,38 and in 3 other studies a seizure reduction was already observed at some point during chemotherapy.12,36,39 In the remaining 4 articles that described seizure frequency, seizures were assessed at fixed intervals (2 studies)41,42 or at a specific point in time from the start of chemotherapy (2 studies).37,40 The other 4 of 14 studies used seizure control or Engel class as the seizure outcome measure. In these studies, improved seizure control was reported in 30%–100% of cases.22,34,42,43
Pace and colleagues41 prospectively assessed seizure frequency after every 3 TMZ cycles in patients with progressive LGG and uncontrolled epilepsy. They found a ≥50% seizure reduction in 48% of patients.41 A prospective study in 30 patients with progressive LGG described that 8/13 patients (62%) with previously intractable seizures showed a reduction in seizure frequency after the beginning of TMZ treatment.24 In a retrospective cohort of 50 patients with LGG and uncontrolled epilepsy who were on a stable AED dose, 44% showed a ≥50% seizure reduction 6 months after the start of TMZ.40 In this cohort, the presence of focal neurological symptoms appeared to be positively associated with a ≥50% seizure reduction. In addition, seizure reduction was an independent prognostic factor for progression-free and overall survival.40 In another small series involving astrocytoma treated with PCV, all 8 patients with pharmacoresistant epilepsy had a seizure reduction after the second course of chemotherapy.37 The largest prospective study in which 149 patients with LGG treated with TMZ had been included showed a ≥50% seizure reduction in 58%, although patients' seizure status before TMZ treatment was not specified.23
In the only study with a control group, a cohort of 39 patients treated with TMZ was retrospectively compared with 30 patients with LGG under observation.12 A ≥50% seizure reduction was observed in 59% of the TMZ group in contrast to 13% in the control group. However, when only patients without AED changes were taken into account, seizure reduction was 18% and 0%, respectively.12
Seizure Outcome in Relation to Radiological Responses
We found 8 articles in which radiological responses on MRI were described in relation to seizure outcome (Table 3).12,13,27,34,36,38,40,41 All articles included patients with WHO grade II glioma. Five studies reported on radiological response after TMZ,12,36,38,40,41 1 study on response after PCV,34 and 2 studies on the radiological response after focal irradiation.13,27 In 6 studies, data on seizure reduction were available in patients with and without an objective response on MRI.12,13,27,36,40,41
Table 3.
Summary of radiological responses in relation to seizure outcome
| Article | Treatment | Time of Response Assessment | Radiological Response, n | Related Seizure Outcome |
|---|---|---|---|---|
| Rogers et al, 199327 | Focal fractionated irradiation | N/S | 3/5 (60%) | Patients with medically intractable epilepsy: >75% seizure reduction in 3 patients with PR and in 1 patient with SD |
| Brada et al, 200336 | TMZ | Every 3 m in year 1, every 6 m in year 2–3; maximum response was assessed after a median of 12–15 m | 17/29 (59%) | Patients with history of epilepsy: seizure reduction in 10/15 (67%) with response on MRI and in 4/12 (33%) without a response on MRI |
| Pace et al, 200341 | TMZ | Every 3 treatment cycles with a median duration of response of 10 m | 24/43 (56%) | Patients with previously uncontrolled epilepsy: seizure reduction in 8/12 patients (67%) with CR or PR on MRI and in 7/14 patients (50%) with SD on MRI |
| Hoang-Xuan et al, 200438 | TMZ | After median follow-up of 14 m (range 6–46 m) | 18/59 (31%) | Neurological improvement (eg, a seizure reduction) in 12/36 (33%) with stable disease on MRI |
| Biemond-ter Stege et al, 200534 | PCV chemotherapy | N/S | 16/20 (80%) | Improved seizure control in most of 16 patients showing radiological response to treatment |
| Sherman et al, 201112 | TMZ | N/S | N/S | Seizure reduction in 1/23 patients with an MRI response. All other patients with a seizure reduction had SD on MRI |
| Ruda et al, 201313 | Focal fractionated irradiation | After 3 m After 6 m After 12 m |
18/43 (42%) 14/34 (41%) N/S |
Seizure reduction in 15/18 (83%) with response on MRI and 16/25 (64%) without a response on MRI Seizure reduction in 12/14 (86%) with response on MRI and 14/20 (70%) without a response on MRI Seizure reduction in 78% with response on MRI and 76% without a response on MRI |
| Koekkoek et al, 201440 | TMZ | After 6 m | 15/62 (24%) | Seizure reduction in 8/28 (29%) with response on MRI and in 7/34 (21%) without a response on MRI |
Abbreviations: CR, complete response; PR, partial response; SD, stable disease; PCV, procarbazine, lomustine, and vincristine; N/S, not specified; m, months.
Four studies that evaluated the response after TMZ applied the revised Macdonald criteria to assess MRI response.17,36,38,40,41,44 In 3 of these studies, patients with and without a response on MRI were compared in terms of seizure reduction.36,40,41 In all 3 studies, the percentage of patients with a seizure reduction was higher among those showing an MRI response than in those without. Nevertheless, 21%–50% of patients with stable disease on MRI still experienced seizure reduction after TMZ treatment, compared with 29%–67% of patients with an objective radiological response.36,40,41 The timing of the response assessment differed considerably between studies, ranging from a single response assessment at a fixed time point40 to a series of assessments every 3–6 months.36,41 One other study on TMZ showed a neurological improvement in 33% of patients with radiologically stable disease (SD), although the precise number of patients with a seizure reduction was not reported.38 The only study in PCV-treated patients reported improved seizure control in most, with a response on MRI, but seizure control was not reported in patients without an objective radiological response.34
The largest study on radiological response after radiotherapy analyzing both WHO grades II and III glioma patients applied the revised Macdonald criteria for LGG as well.13 At 3, 6, and 12 months after radiotherapy the percentage of patients with a ≥50% seizure reduction was highest in the group with an objective radiological response, ranging from 78% to 86%, compared with 64%–76% of patients with SD on MRI.13 In a series of 5 cases, 3 patients with a partial response on CT or MRI showed a >75% seizure reduction. Of the 2 remaining patients with radiological SD, 1 patient had a reduction in seizure frequency.27
Discussion
All studies that we included in this systematic review demonstrated improvements in the seizure status of patients with LGG after radiotherapy or chemotherapy. In the largest patient series, ≥50% seizure reduction between 44% and 77% has been reported after focal fractionated irradiation and TMZ chemotherapy.13,40 In general, a seizure reduction appeared to be more common in patients with an objective radiological response. However, in all studies that reported on imaging response in relation to seizure outcome, a substantial part of patients with SD reported a seizure reduction as well. These findings underscore the importance of monitoring patients' seizure status, as a seizure reduction may be the only noticeable effect of antitumor treatment.
Many studies have shown that tumor resection may positively influence seizure outcome in LGG patients.6,11,45–47 In 13/14 studies on chemotherapy and in 4/10 studies on radiotherapy, part of the included patients underwent previous surgery. Due to a possibly long-term positive effect of tumor resection on seizures, seizure reduction due to radio- or chemotherapy might have been overestimated. Furthermore, the stage of the disease course at the time of analysis differed considerably both between and within the studies.
There are additional limitations of this review that are mostly inherent to the diversity of studies. In most cases, a clear definition of measures such as seizure control or seizure reduction was lacking. As patients' seizure status was often not the primary outcome measure, in many of the studies little information was available both on seizures before the start of antitumor treatment and on seizure outcome. In the subset of studies in which well-defined seizure outcome measures were available, these measures were not consistent. Thus, there is class III evidence at best regarding seizure outcome after antitumor treatment, since most studies had a retrospective nature and were lacking an appropriate control group. In addition, data regarding concomitant AED use were lacking in almost all studies. Apart from 3 studies in which patients on a stable AED dose were analyzed,12,13,40 a change in AED dose and/or AED type could have underlain the improved seizure outcome. Due to publication bias, the true effect of antitumor treatment on epilepsy may be overestimated as well, as a tendency to report only the positive effects of antitumor treatment might be expected. Lastly, we evaluated the effect of different types of irradiation and varying chemotherapy schedules in one review, whereas different regimens may have diverse effects on seizure control.15 Altogether, caution in interpreting and comparing the data is necessary.
Nevertheless, our results strongly suggest that radiotherapy as well as chemotherapy have a positive effect on seizure control. In determining the effect of antitumor treatment, gaining insight into seizure outcome is of major clinical importance, as a decrease in seizure burden could contribute to an improvement in patients' quality of life.8 However, it should be noted that a reduction in seizure frequency will not necessarily lead to a clinically significant benefit for the patient. In 2 studies on non–tumor related epilepsy, patients without complete seizure freedom and patients with a <90% seizure reduction reported significantly worse scores on quality of life subscales compared with patients who achieved complete seizure freedom.48,49 Moreover, a reduction in generalized seizures may be clinically more relevant than a similar reduction in simple partial seizures. Therefore, seizure outcome measures should preferably be used in combination with other symptom burden or quality-of-life instruments. Given the wide range of seizure outcome measures that have been applied so far, more uniform measures are highly needed to further determine the clinical relevance of an improved seizure outcome in glioma patients.
Smaller retrospective studies suggest that AED withdrawal can successfully be applied in a selected group of brain tumor patients—for example, where postoperative seizures are absent or patients have extratemporal located tumors.50,51 Reducing AED use in case of seizure control may decrease the risk of drug toxicity and improve neurocognitive functioning.9,52,53 The clinical relevance of keeping track of patients' seizure status particularly applies to patients with WHO grade II glioma with favorable prognostic features such as a 1p/19q codeletion and to patients in whom seizures are the only sign of a tumor.3 In case antitumor treatment leads to long-term seizure freedom in these patients, reduction or (when possible) withdrawal of AEDs should seriously be considered.54
Although the data are scarce, the discrepancies between seizure outcome and the observed radiological response demonstrate that imaging alone does not seem to be fully representative of the effects of antitumor treatment. This is illustrated by the finding that 21%–50% of patients with WHO grade II glioma and SD on MRI experienced a seizure reduction after treatment with TMZ.36,40,41 Similar criteria were applied to assess radiological response in these studies; however, 2 studies used older, probably less sensitive MRI techniques.36,41 In a recent study, more than 60% of patients with radiological SD showed a seizure reduction after radiotherapy.13 These findings suggest that the observed response on MRI underestimates the benefit of the treatment.19Although imaging is a regular part of the follow-up in LGG patients, the relation between imaging response and survival is unclear, and the observed radiological response seems to depend on the timing of the assessment.17,19 In patients with grade II or III who underwent radiotherapy, for example, the maximum response on MRI was assessed after 3 months.13 However, in a cohort of 33 patients, a prolonged radiological response after radiotherapy was observed that lasted for years.55 Similar long-term responses were seen in patients treated with PCV.56 In a cohort of 149 patients treated with up-front TMZ, the time to maximum response ranged widely from 3 to 30 months (median 12 mo).23 So the imaging responses are regularly delayed, which emphasizes the relevance of a complementary role of seizure outcome in evaluating the effect of antitumor treatment. Interestingly, one of the retrospective studies on seizures after TMZ treatment demonstrated that a seizure reduction after 6 months was an independent prognostic factor for both progression-free and overall survival in patients with LGG, in contrast to the radiological response.40 Such findings suggest that seizure outcome may even serve as a surrogate marker for tumor response. Of note, this does not necessarily imply that patients' seizure status could serve as a general marker for tumor behavior. Although seizures in LGG patients rarely present during a stable course of disease, the association between an increased seizure frequency and tumor recurrence is controversial.6,11,13,40,52,57 Moreover, seizures sometimes occur as an acute complication during antitumor treatment.58–62
The precise molecular biological mechanism through which radiotherapy and chemotherapy contribute to improved seizure control still needs to be clarified. Remarkably, in a cohort of 143 patients receiving stereotactic radiotherapy, a decrease in seizure prevalence was observed already at 6 weeks after treatment.25 A similar early reduction was observed in a patient with medically intractable epilepsy treated with TMZ.63 Together with the fact that seizure frequency reduces in the absence of a response on MRI, these observations suggest that improved seizure control after either radiotherapy or chemotherapy cannot be attributed to a reduction in tumor size. Probably, molecular changes in the peritumoral microenvironment directly resulting from antitumor therapy underlie the seizure reduction, although current evidence is limited.63,64 TMZ is thought to reduce the intrinsic epileptogenicity of the tumor through a decrease in glutamate levels released from glioma cells.65 A downregulation of glutamate receptors is also associated with an increased survival in glioma patients treated with TMZ.66 Other changes in the microenvironment of the tumor might play a role as well—for example, regarding the synthesis of neurotransmitters and inhibition of the immune response.67,68 After brachytherapy, an increased benzodiazepine receptor density in the brain adjacent to the tumor was found in patients with a significant seizure reduction.33 In another study, a dose-dependent rate of seizure improvement was found after gamma knife surgery, suggesting that higher radiation doses are possibly more effective in reducing the epileptogenicity of cortical structures around the tumor.15,29
The rate of seizure frequency reduction also appeared to depend on the timing of radiotherapy, although we found contradictory results.13,32 Nonetheless, a decrease in tumor size, albeit small, could still be the mechanism of action leading to a seizure reduction. After all, the first 25% decrease in the area of the tumor does not qualify for an objective response according to the current RANO criteria.17 Some patients will therefore be regarded as nonresponders, despite a modest reduction in tumor size.
In conclusion, this systematic review demonstrates the improvements in patients' seizure status that occur after radiotherapy and chemotherapy, as well as the discrepancies between seizure outcome and the radiological response. Improved seizure control not only implies a direct clinical benefit for the patient, but may be a sign that the tumor responds to treatment. Therefore, our findings highlight the importance of using seizure outcome along with radiological response in evaluating the effect of antitumor treatment, particularly in patients with LGG. Given the current lack of high-quality studies, future randomized controlled studies are needed to confirm the positive effect of radiotherapy and chemotherapy on seizure frequency. Preferably, these studies should focus on the additional value of patients' seizure status and other clinical outcome measures in assessing tumor response, as well as their prognostic significance for survival.
Funding
Johan A. F. Koekkoek and Martin J. B. Taphoorn received funds from the St. Jacobusstichting The Hague, Foundation ZOLEON and Foundation Chanrone.
Conflict of interest statement. The authors report no conflict of interest.
Supplementary Material
References
- 1. Danfors T, Ribom D, Berntsson SG, et al. Epileptic seizures and survival in early disease of grade 2 gliomas. Eur J Neurol. 2009;16(7):823–831. [DOI] [PubMed] [Google Scholar]
- 2. Weller M, Stupp R, Wick W. Epilepsy meets cancer: when, why, and what to do about it? Lancet Oncol. 2012;13(9):e375–e382. [DOI] [PubMed] [Google Scholar]
- 3. Kerkhof M, Vecht CJ. Seizure characteristics and prognostic factors of gliomas. Epilepsia. 2013;54(Suppl 9):12–17. [DOI] [PubMed] [Google Scholar]
- 4. Leighton C, Fisher B, Bauman G, et al. Supratentorial low-grade glioma in adults: an analysis of prognostic factors and timing of radiation. J Clin Oncol. 1997;15(4):1294–1301. [DOI] [PubMed] [Google Scholar]
- 5. Piepmeier J, Christopher S, Spencer D, et al. Variations in the natural history and survival of patients with supratentorial low-grade astrocytomas. Neurosurgery. 1996;38(5):872–878. [DOI] [PubMed] [Google Scholar]
- 6. You G, Sha ZY, Yan W, et al. Seizure characteristics and outcomes in 508 Chinese adult patients undergoing primary resection of low-grade gliomas: a clinicopathological study. Neuro Oncol. 2012;14(2):230–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smits A, Duffau H. Seizures and the natural history of World Health Organization grade II gliomas: a review. Neurosurgery. 2011;68(5):1326–1333. [DOI] [PubMed] [Google Scholar]
- 8. Klein M, Engelberts NH, van der Ploeg HM, et al. Epilepsy in low-grade gliomas: the impact on cognitive function and quality of life. Ann Neurol. 2003;54(4):514–520. [DOI] [PubMed] [Google Scholar]
- 9. Taphoorn MJ, Klein M. Cognitive deficits in adult patients with brain tumours. Lancet Neurol. 2004;3(3):159–168. [DOI] [PubMed] [Google Scholar]
- 10. van Breemen MS, Wilms EB, Vecht CJ. Epilepsy in patients with brain tumours: epidemiology, mechanisms, and management. Lancet Neurol. 2007;6(5):421–430. [DOI] [PubMed] [Google Scholar]
- 11. Chang EF, Potts MB, Keles GE, et al. Seizure characteristics and control following resection in 332 patients with low-grade gliomas. J Neurosurg. 2008;108(2):227–235. [DOI] [PubMed] [Google Scholar]
- 12. Sherman JH, Moldovan K, Yeoh HK, et al. Impact of temozolomide chemotherapy on seizure frequency in patients with low-grade gliomas. J Neurosurg. 2011;114(6):1617–1621. [DOI] [PubMed] [Google Scholar]
- 13. Ruda R, Magliola U, Bertero L, et al. Seizure control following radiotherapy in patients with diffuse gliomas: a retrospective study. Neuro Oncol. 2013;15(12):1739–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kahlenberg CA, Fadul CE, Roberts DW, et al. Seizure prognosis of patients with low-grade tumors. Seizure. 2012;21(7):540–545. [DOI] [PubMed] [Google Scholar]
- 15. Quigg M, Barbaro NM. Stereotactic radiosurgery for treatment of epilepsy. Arch Neurol. 2008;65(2):177–183. [DOI] [PubMed] [Google Scholar]
- 16. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: Response Assessment in Neuro-Oncology working group. J Clin Oncol. 2010;28(11):1963–1972. [DOI] [PubMed] [Google Scholar]
- 17. van den Bent MJ, Wefel JS, Schiff D, et al. Response Assessment in Neuro-Oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583–593. [DOI] [PubMed] [Google Scholar]
- 18. Ricard D, Kaloshi G, Amiel-Benouaich A, et al. Dynamic history of low-grade gliomas before and after temozolomide treatment. Ann Neurol. 2007;61(5):484–490. [DOI] [PubMed] [Google Scholar]
- 19. Butowski N, Chang SM. Endpoints for clinical trials and revised assessment in neuro-oncology. Curr Opin Neurol. 2012;25(6):780–785. [DOI] [PubMed] [Google Scholar]
- 20. Viaccoz A, Lekoubou A, Ducray F. Chemotherapy in low-grade gliomas. Curr Opin Oncol. 2012;24(6):694–701. [DOI] [PubMed] [Google Scholar]
- 21. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 22. Mason WP, Krol GS, Deangelis LM. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996;46(1):203–207. [DOI] [PubMed] [Google Scholar]
- 23. Kaloshi G, Benouaich-Amiel A, Diakite F, et al. Temozolomide for low-grade gliomas: predictive impact of 1p/19q loss on response and outcome. Neurology. 2007;68(21):1831–1836. [DOI] [PubMed] [Google Scholar]
- 24. Tosoni A, Franceschi E, Ermani M, et al. Temozolomide three weeks on and one week off as first line therapy for patients with recurrent or progressive low grade gliomas. J Neurooncol. 2008;89(2):179–185. [DOI] [PubMed] [Google Scholar]
- 25. Plathow C, Schulz-Ertner D, Thilman C, et al. Fractionated stereotactic radiotherapy in low-grade astrocytomas: long-term outcome and prognostic factors. Int J Radiat Oncol Biol Phys. 2003;57(4):996–1003. [DOI] [PubMed] [Google Scholar]
- 26. Shankar A, Rajshekhar V. Radiological and clinical outcome following stereotactic biopsy and radiotherapy for low-grade insular astrocytomas. Neurol India. 2003;51(4):503–506. [PubMed] [Google Scholar]
- 27. Rogers LR, Morris HH, Lupica K. Effect of cranial irradiation on seizure frequency in adults with low-grade astrocytoma and medically intractable epilepsy. Neurology. 1993;43(8):1599–1601. [DOI] [PubMed] [Google Scholar]
- 28. Rossi GF, Scerrati M, Roselli R. Epileptogenic cerebral low-grade tumors: effect of interstitial stereotactic irradiation on seizures. Appl Neurophysiol. 1985;48(1–6):127–132. [DOI] [PubMed] [Google Scholar]
- 29. Schröttner O, Eder HG, Unger F, et al. Radiosurgery in lesional epilepsy: brain tumors. Stereotact Funct Neurosurg. 1998;70(Suppl 1):50–56. [DOI] [PubMed] [Google Scholar]
- 30. Schröttner O, Unger F, Eder HG, et al. Gamma-knife radiosurgery of mesiotemporal tumour epilepsy observations and long-term results. Acta Neurochir Suppl. 2002;84:49–55. [DOI] [PubMed] [Google Scholar]
- 31. Scerrati M, Montemaggi P, Iacoangeli M, et al. Interstitial brachytherapy for low-grade cerebral gliomas: analysis of results in a series of 36 cases. Acta Neurochir (Wien). 1994;131(1–2):97–105. [DOI] [PubMed] [Google Scholar]
- 32. van den Bent MJ, Afra D, de Witte O, et al. Long-term efficacy of early versus delayed radiotherapy for low-grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet. 2005;366(9490):985–990. [DOI] [PubMed] [Google Scholar]
- 33. Warnke PC, Berlis A, Weyerbrock A, et al. Significant reduction of seizure incidence and increase of benzodiazepine receptor density after interstitial radiosurgery in low-grade gliomas. Acta Neurochir Suppl. 1997;68:90–92. [DOI] [PubMed] [Google Scholar]
- 34. Biemond-ter Stege EM, Kros JM, de Bruin HG, et al. Successful treatment of low-grade oligodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005;103(4):802–809. [DOI] [PubMed] [Google Scholar]
- 35. Blonski M, Taillandier L, Herbet G, et al. Combination of neoadjuvant chemotherapy followed by surgical resection as a new strategy for WHO grade II gliomas: a study of cognitive status and quality of life. J Neurooncol. 2012;106(2):353–366. [DOI] [PubMed] [Google Scholar]
- 36. Brada M, Viviers L, Abson C, et al. Phase II study of primary temozolomide chemotherapy in patients with WHO grade II gliomas. Ann Oncol. 2003;14(12):1715–1721. [DOI] [PubMed] [Google Scholar]
- 37. Frenay MP, Fontaine D, Vandenbos F, et al. First-line nitrosourea-based chemotherapy in symptomatic non-resectable supratentorial pure low-grade astrocytomas. Eur J Neurol. 2005;12(9):685–690. [DOI] [PubMed] [Google Scholar]
- 38. Hoang-Xuan K, Capelle L, Kujas M, et al. Temozolomide as initial treatment for adults with low-grade oligodendrogliomas or oligoastrocytomas and correlation with chromosome 1p deletions. J Clin Oncol. 2004;22(15):3133–3138. [DOI] [PubMed] [Google Scholar]
- 39. Lebrun C, Fontaine D, Bourg V, et al. Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol. 2007;14(4):391–398. [DOI] [PubMed] [Google Scholar]
- 40. Koekkoek JA, Dirven L, Heimans JJ, et al. Seizure reduction in a low-grade glioma: more than a beneficial side effect of temozolomide. J Neurol Neurosurg Psychiatry. 2014; 10.1136/jnnp-2014-308136. [DOI] [PubMed] [Google Scholar]
- 41. Pace A, Vidiri A, Galie E, et al. Temozolomide chemotherapy for progressive low-grade glioma: clinical benefits and radiological response. Ann Oncol. 2003;14(12):1722–1726. [DOI] [PubMed] [Google Scholar]
- 42. Soffietti R, Ruda R, Bradac GB, et al. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998;43(5):1066–1073. [DOI] [PubMed] [Google Scholar]
- 43. Taillandier L, Duffau H. Epilepsy and insular Grade II gliomas: an interdisciplinary point of view from a retrospective monocentric series of 46 cases. Neurosurg Focus. 2009;27(2):E8. [DOI] [PubMed] [Google Scholar]
- 44. Macdonald DR, Cascino TL, Schold SC, Jr, et al. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. [DOI] [PubMed] [Google Scholar]
- 45. Luyken C, Blumcke I, Fimmers R, et al. The spectrum of long-term epilepsy-associated tumors: long-term seizure and tumor outcome and neurosurgical aspects. Epilepsia. 2003;44(6):822–830. [DOI] [PubMed] [Google Scholar]
- 46. Chaichana KL, Parker SL, Olivi A, et al. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111(2):282–292. [DOI] [PubMed] [Google Scholar]
- 47. Pallud J, Audureau E, Blonski M, et al. Epileptic seizures in diffuse low-grade gliomas in adults. Brain. 2014;137(Pt 2):449–462. [DOI] [PubMed] [Google Scholar]
- 48. Birbeck GL, Hays RD, Cui X, et al. Seizure reduction and quality of life improvements in people with epilepsy. Epilepsia. 2002;43(5):535–538. [DOI] [PubMed] [Google Scholar]
- 49. McLachlan RS, Rose KJ, Derry PA, et al. Health-related quality of life and seizure control in temporal lobe epilepsy. Ann Neurol. 1997;41(4):482–489. [DOI] [PubMed] [Google Scholar]
- 50. Khan RB, Onar A. Seizure recurrence and risk factors after antiepilepsy drug withdrawal in children with brain tumors. Epilepsia. 2006;47(2):375–379. [DOI] [PubMed] [Google Scholar]
- 51. Das RR, Artsy E, Hurwitz S, et al. Outcomes after discontinuation of antiepileptic drugs after surgery in patients with low grade brain tumors and meningiomas. J Neurooncol. 2012;107(3):565–570. [DOI] [PubMed] [Google Scholar]
- 52. Vecht CJ, Kerkhof M, Duran-Pena A. Seizure prognosis in brain tumors: new insights and evidence-based management. Oncologist. 2014;19(7):751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Taphoorn MJ. Neurocognitive sequelae in the treatment of low-grade gliomas. Semin Oncol. 2003;30(6 Suppl 9):45–48. [DOI] [PubMed] [Google Scholar]
- 54. Koekkoek JA, Kerkhof M, Dirven L, et al. Withdrawal of antiepileptic drugs in glioma patients after long-term seizure freedom: design of a prospective observational study. BMC Neurol. 2014;14:157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pallud J, Llitjos JF, Dhermain F, et al. Dynamic imaging response following radiation therapy predicts long-term outcomes for diffuse low-grade gliomas. Neuro Oncol. 2012;14(4):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peyre M, Cartalat-Carel S, Meyronet D, et al. Prolonged response without prolonged chemotherapy: a lesson from PCV chemotherapy in low-grade gliomas. Neuro Oncol. 2010;12(10):1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Rosati A, Buttolo L, Stefini R, et al. Efficacy and safety of levetiracetam in patients with glioma: a clinical prospective study. Arch Neurol. 2010;67(3):343–346. [DOI] [PubMed] [Google Scholar]
- 58. Alexander E, III, Loeffler JS. Radiosurgery for primary malignant brain tumors. Semin Surg Oncol. 1998;14(1):43–52. [DOI] [PubMed] [Google Scholar]
- 59. Della PA, Denaro L, Rossetto M, et al. Postoperative seizure in high grade glioma patients treated with BCNU wafers. A mono-institutional experience. J Neurooncol. 2011;105(2):275–280. [DOI] [PubMed] [Google Scholar]
- 60. Waters JD, Rose B, Gonda DD, et al. Immediate post-operative brachytherapy prior to irradiation and temozolomide for newly diagnosed glioblastoma. J Neurooncol. 2013;113(3):467–477. [DOI] [PubMed] [Google Scholar]
- 61. Shrieve DC, Alexander E, III, Wen PY, et al. Comparison of stereotactic radiosurgery and brachytherapy in the treatment of recurrent glioblastoma multiforme. Neurosurgery. 1995;36(2):275–282. [DOI] [PubMed] [Google Scholar]
- 62. Chin LS, Lazio BE, Biggins T, et al. Acute complications following gamma knife radiosurgery are rare. Surg Neurol. 2000;53(5):498–502. [DOI] [PubMed] [Google Scholar]
- 63. Hu A, Xu Z, Kim RY, et al. Seizure control: a secondary benefit of chemotherapeutic temozolomide in brain cancer patients. Epilepsy Res. 2011;95(3):270–272. [DOI] [PubMed] [Google Scholar]
- 64. Ruda R, Trevisan E, Soffietti R. Epilepsy and brain tumors. Curr Opin Oncol. 2010;22(6):611–620. [DOI] [PubMed] [Google Scholar]
- 65. de Groot M, Reijneveld JC, Aronica E, et al. Epilepsy in patients with a brain tumour: focal epilepsy requires focused treatment. Brain. 2012;135(Pt 4):1002–1016. [DOI] [PubMed] [Google Scholar]
- 66. Ciceroni C, Bonelli M, Mastrantoni E, et al. Type-3 metabotropic glutamate receptors regulate chemoresistance in glioma stem cells, and their levels are inversely related to survival in patients with malignant gliomas. Cell Death Differ. 2013;20(3):396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Litterman AJ, Zellmer DM, Grinnen KL, et al. Profound impairment of adaptive immune responses by alkylating chemotherapy. J Immunol. 2013;190(12):6259–6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Shamji MF, Fric-Shamji EC, Benoit BG. Brain tumors and epilepsy: pathophysiology of peritumoral changes. Neurosurg Rev. 2009;32(3):275–284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.