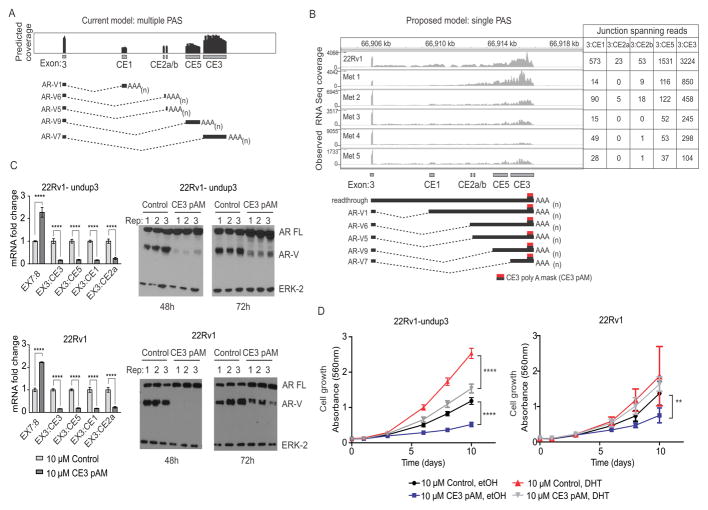
Figure 2.
Blockade of the AR CE3 PAS inhibits AR-V expression. A. Schematic of predicted AR RNA-seq coverage based on use of annotated splice junctions and PASs in AR cryptic exons (CEs). B. Observed AR RNA-seq coverage from 22Rv1 and metastatic biopsies (top) consistent with a new model wherein multiple AR-Vs arise from use of multiple splice acceptor sites that terminate at a single PAS (bottom). The location at which CE3 pAM morpholino binds to induce steric blockade is shown. C. Left panels: 22Rv1-undup3 (top) and 22Rv1 (bottom) cells were transfected with 10 μM control or CE3-pAM. Mean mRNA fold change of AR and AR-Vs relative to actin control is shown (n=6, error bars=95% confidence intervals). **** p≤0.0001, unpaired t-tests. Right panels: Western blot of lysates from transfected cells, probed with antibodies to AR NTD or ERK-2 (loading control). D. Crystal violet staining to assess growth of 22Rv1-undup3 (left) and 22Rv1 (right) cells transfected as in panel C maintained in androgen-deplete medium (CSS) in the presence of 1 nM DHT or vehicle control (ethanol). Data points represent means (n=6, 22Rv1-undup1, n=4, 22Rv1; error bars = 95% confidence intervals). **** p≤ 0.0001, ** p ≤ 0.01, ns p>0.05 p-values in a two-tailed t-test.