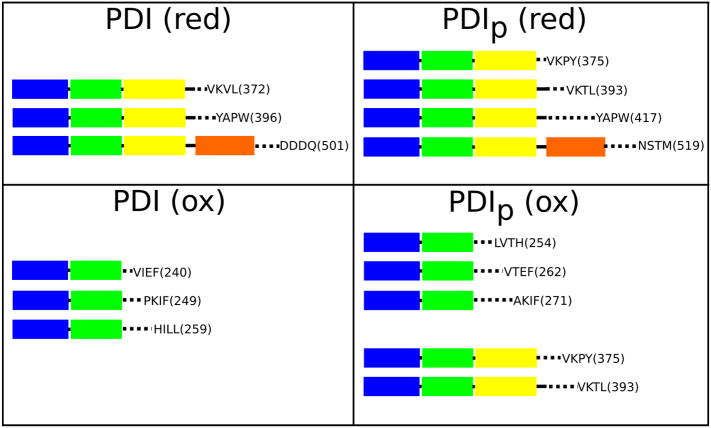
Fig. 6.
Limited proteolysis reveals differences in flexibility between reduced and oxidized hPDI.
Recombinant human PDI and its close homolog, the pancreas-specific form PDIp, were subjected to limited digestion by chymotrypsin in reducing and in oxidizing conditions. The proteins (15 μM) were incubated with chymotrypsin (75 nM) at pH 7.6, 37 °C for 120 min in presence either of 10 mM dithiothreitol or 10 mM diamide. The digestion mixes were subjected to electrospray ionization mass spectrometry, analyzing products of mass > 20 kDa (see Walker [66]). All the products detected were found to contain the intact a-b region despite the presence of multiple potential chymotryptic cleavage sites in these domains. Products are identified in the figure by their C-terminal residue (i.e. the site at which enzymic cleavage has occurred, numbering as for the unprocessed translation product) and intact domains are coloured according to the scheme of Fig. 1. In reducing conditions all the products contain intact abb′x domains with cleavage occurring only at sites within a′ and c domains; in oxidizing conditions the fragments are smaller with most fragments deriving from cleavage within the b′ domain although, for PDIp, there are two products found in both reducing and oxidizing conditions.