Fig. 8.
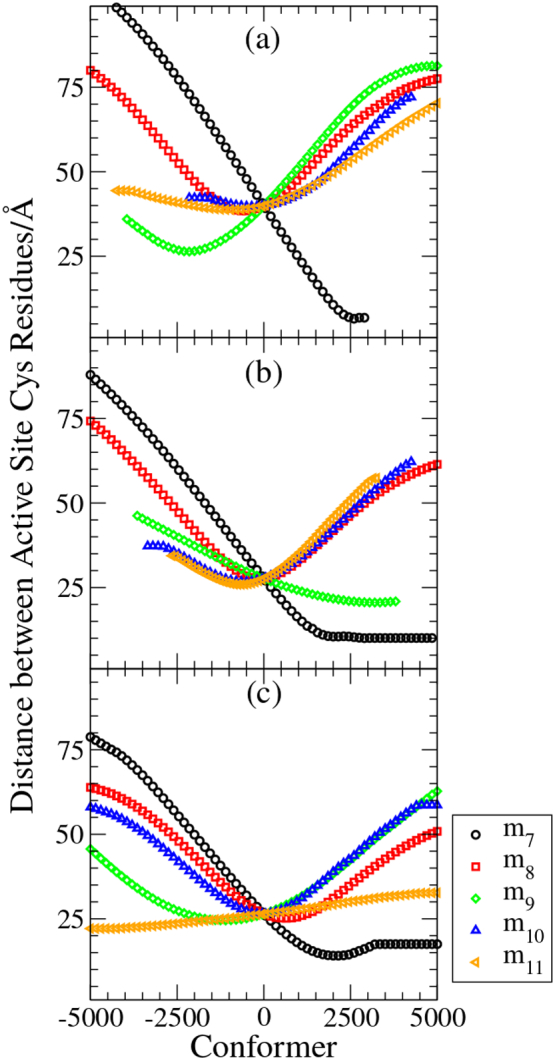
Variation in distance between active site Cys residues through flexible motion.
Distances between the α-carbon atoms of the exposed active site Cys residues in the a and a′ domains were determined through simulations of the lowest frequency flexible modes of motion. Conformer zero represents the initial structure observed in the crystal structure of a) oxidized human PDI (4ELI), b) reduced human PDI (4EKZ) and c) yeast PDI (2B5E). Conformers to + 5000 and − 5000 represent structures determined through the flexibility simulations in modes m7 to m11. For most of the flexible modes, the combination of tilting and twisting of domains ensures that the distance between sites can both increase or decrease (see Fig. 7). But m7 corresponds to domains a and a′ moving towards (+ ve conformers) and away from (− ve conformers) each other, so the distance between active sites declines to low values in the + ve conformers as the domains approach; in b) and c) protein structural constraints limit the closest approach and the simulations show that a minimum distance is defined as the simulations progress to generate conformers >+2000, whereas in a) (oxidized human PDI) there are fewer structural constraints and the simulation progresses until steric contact is made between the approaching domains, at which point the simulation halts.
