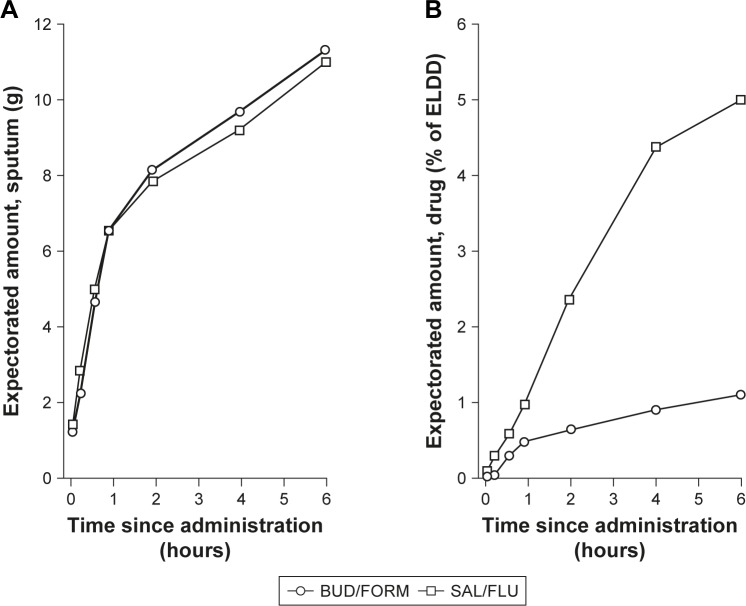
Figure 1.
Cumulative mean amounts of expectorated sputum (A) and budesonide and fluticasone propionate (B) over 6-hour collection after inhalation of a dose of salmeterol/fluticasone propionate (50/500 µg via Diskus®; GlaxoSmithKline, Brentford, UK) or budesonide/formoterol (400/12 µg via Turbuhaler®; AstraZeneca, Gothenburg, Sweden). Mean value plots of the amount of (A) expectorated sputum (arithmetic means) and (B) budesonide and fluticasone propionate in the expectorated sputum (percentage of ELDD, geometric means), cumulative over the 6-hour collection period.
Notes: Reproduced from Dalby C, Polanowski T, Larsson T, Borgstrom L, Edsbacker S, Harrison TW. The bioavailability and airway clearance of the steroid component of budesonide/formoterol and salmeterol/fluticasone after inhaled administration in patients with COPD and healthy subjects: a randomized controlled trial. Respir Res. 2009;10:104. Copyright ©2009 Dalby et al; licensee BioMed Central Ltd. Creative commons license: https://creativecommons.org/licenses/by/2.0/legalcode.34
Abbreviations: BUD/FORM, budesonide/formoterol; ELDD, estimated lung-deposited dose; SAL/FLU, salmeterol/fluticasone propionate.