Figure 6.
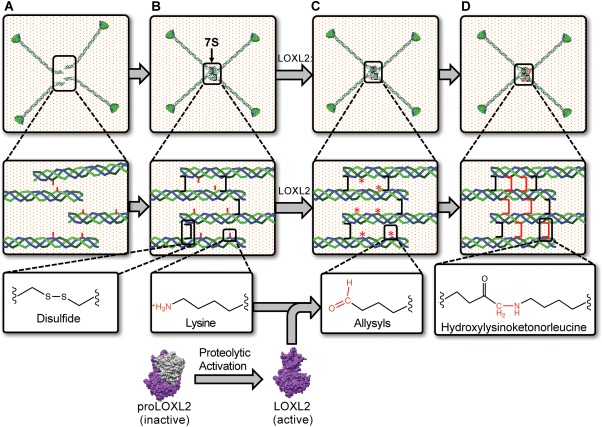
LOXL2 mediated crosslinking of 7S dodecamer. The assembly of four triple helical protomers occurs in the extracellular space. 7S domains contain intrinsic sequences that drive their assembly into a tetramer of trimers that is dodecameric. (A) The 7S dodecamer is stabilized by intramolecular (not shown) and intermolecular (black bracket) disulfide crosslinks. (B) As collagen IV networks are deposited into the BM, proteolytically activated LOXL268 promotes the formation of lysyl‐derived crosslinks in the 7S dodecamer (C) by catalysis of the oxidative deamination of the ɛ‐amino group of lysyl residues (red sticks), generating allysine (*). Allysines spontaneously form crosslinks (red brackets) with neighboring lysyl or hydroxylysyl residues. (D) As hydroxylysine is abundant in collagen IV, hydroxylysyl‐derived crosslinks such as hydroxylysinoketonorleucine is likely to be formed in 7S dodecamer. The occurrence of intra‐ and intermolecular crosslinks in the 7S dodecamer, including disulfides and LOXL2‐mediated hydroxylysinoketonorleucine, provides collagen IV scaffolds with the required strength to stabilize BMs.
