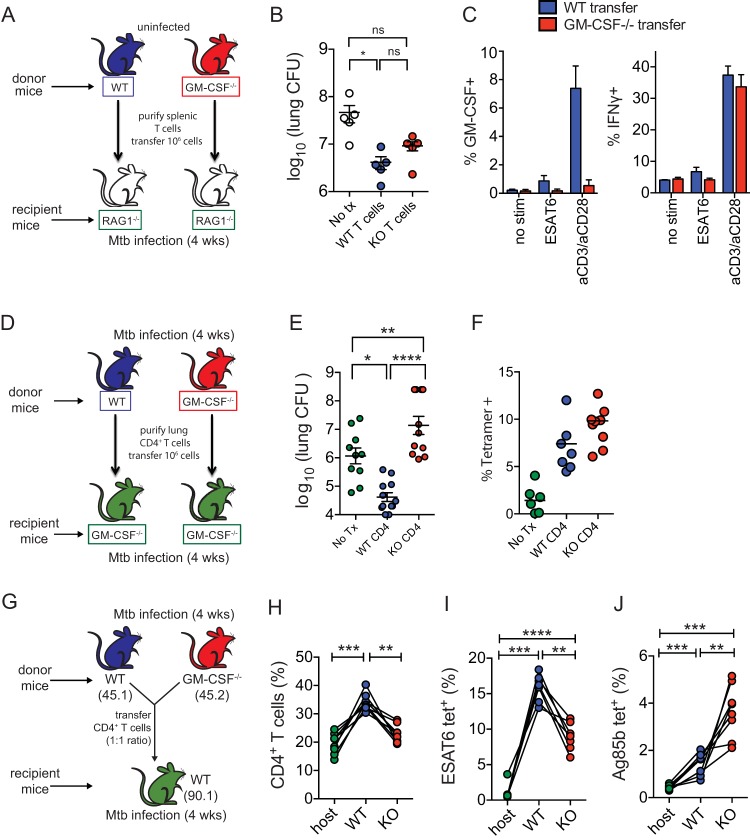
FIG 4 .
T cell-derived GM-CSF controls M. tuberculosis growth. (A) Experimental strategy for transfer of donor WT or GM-CSF−/− T cells into RAG−/− recipient mice, followed by M. tuberculosis aerosol infection. (B) CFU in lungs 4 weeks postinfection after WT or GM-CSF−/− T cells were transferred into RAG−/− recipients. Data are representative of five independent experiments. (C) Percentage of CD4+ T cells producing IFN-γ or GM-CSF after 5 h of ESAT61-20 peptide stimulation or anti-CD3/anti-CD28 stimulation with brefeldin A and IL-2 at 4 weeks postinfection. (D) Experimental strategy for adoptive transfer experiments using sublethally irradiated GM-CSF−/− mice as recipients, who were then infected with M. tuberculosis via aerosol. (E) CFU in lungs 4 weeks postinfection after WT or GM-CSF−/− CD4+ T cells were transferred into GM-CSF−/− recipients. Data were compiled from 4 independent infections with a total of n = 16 to 19 mice per condition. (F) Frequency of ESAT63-17 tetramer-positive CD4+ T cells from donor WT and GM-CSF−/− infected mice 4 weeks after sublethal irradiation, adoptive transfer, and aerosol infection. (G) Experimental strategy for adoptive cotransfer of CD4+ T cells from donor WT and GM-CSF−/− Thy1.1 recipients 4 weeks after infection (at a 1:1 ratio) with M. tuberculosis infection via aerosol. (H) Origin of CD4+ T cells based on congenic markers 4 weeks after infection. (I and J) Frequency of ESAT63-17-positive (I) or Ag85b241-256 tetramer-positive (J) CD4+ T cells among total host (e.g., endogenous), WT, or KO CD4+ T cells. Statistical testing was performed by using a paired 1-way ANOVA. *, P < 0.05; **, P < 0.01; ****, P < 0.0001. Error bars indicate SEM.