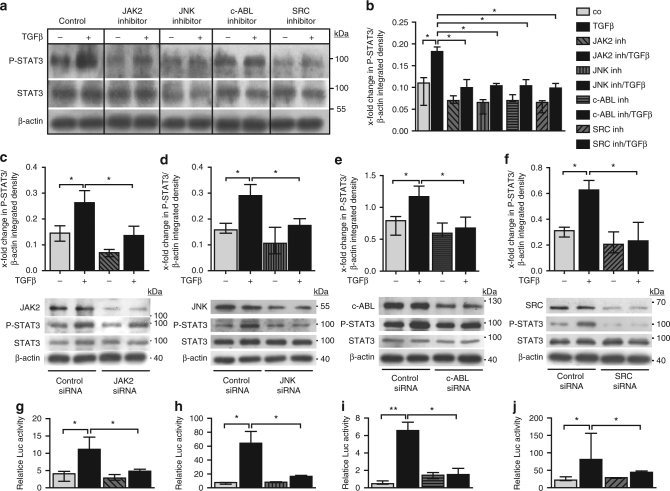
Fig. 4.
Profibrotic kinases pathways converge on P-STAT3 in vitro. a, b Effects of pharmacologic inhibition of JAK2, JNK, c-ABL, and SRC kinases on the levels of P-STAT3 in TGFβ-stimulated fibroblasts in vitro as analyzed by a western blot and b its quantification (n ≥ 4 with 2 technical replicates per condition for all experiments). Representative images and quantification of four independent experiments are shown. c–f Effects of siRNA-mediated knockdown of c JAK2, d JNK, e c-ABL, and f SRC, respectively, on levels of P-STAT3 in healthy human dermal fibroblasts stimulated with TGFβ, as shown by representative western blots and quantifications (n ≥ 4 with 2 technical replicates per group for all experiments). JAK2 (expected molecular size, 132 kDa), JNK (expected molecular size, 49 and 55 kDa), c-ABL (expected molecular size, 125–135 kDa), and SRC (expected molecular size, 60 kDa) are represented by ladders showing 100, 55, 130, and 70 kDa, respectively. Beta-actin expected molecular weight/size is 42 kDa. g–j Relative luciferase activity in fibroblasts transfected with STAT3 luciferase reporter plasmid and treated with g the JAK2 inhibitor TG101209, h the JNK inhibitor SP600125, i the c-ABL inhibitor imatinib mesylate and j the SRC inhibitor SU6656, independently with or without TGFβ stimulation (n = 3 independent experiments with 2 technical replicates per group for all experiments). Luciferase activity was normalized against a non-inducible luciferase construct. Results are shown as median ± interquartile range (IQR). Significance was determined by Mann–Whitney test, as compared to untreated unstimulated fibroblasts or untreated TGFβ-stimulated fibroblasts, respectively. *P < 0.05; **P < 0.01, ***P < 0.001