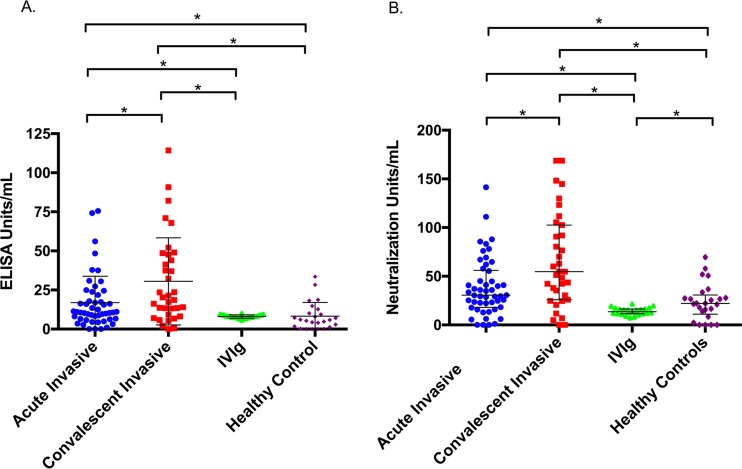
FIG 1.
Binding and functional assessment (antibody-mediated toxin neutralization), adjusted for total IgG per sample. ELISA units and neutralization units were interpolated from a standard 5-PL curve using an anti-LukAB human monoclonal antibody. (A) Median concentration (determined by ELISA) of LukAB binding antibody in each group. Acutely infected subjects contained a median antibody concentration of 10.9 U/ml (IQR, 6.5 to 22.3 U/ml). Subjects in the convalescent phase had a median antibody concentration of 20.3 U/ml (IQR, 9.2 to 47.7 U/ml). IVIg had a median antibody concentration of 8.1 (IQR, 7.3 to 8.9 U/ml). Healthy controls had a median antibody concentration of 5.9 U/ml (IQR, 1.6 to 11.5 U/ml). (B) Median concentration of neutralizing antibody in each group. Sera obtained from acutely infected subjects contained 30.6 U/ml (IQR, 18.1 to 56 U/ml), convalescent-phase sera 54.7 U/ml (IQR, 26 to 102.6 U/ml), IVIg 13.5 U/ml (IQR, 11.6 to 16.5 U/ml), and sera from healthy controls 22.1 U/ml (IQR, 11.1 to 69.6 U/ml). *, P < 0.01.