Figure 2. CT scans of confirmed responders.
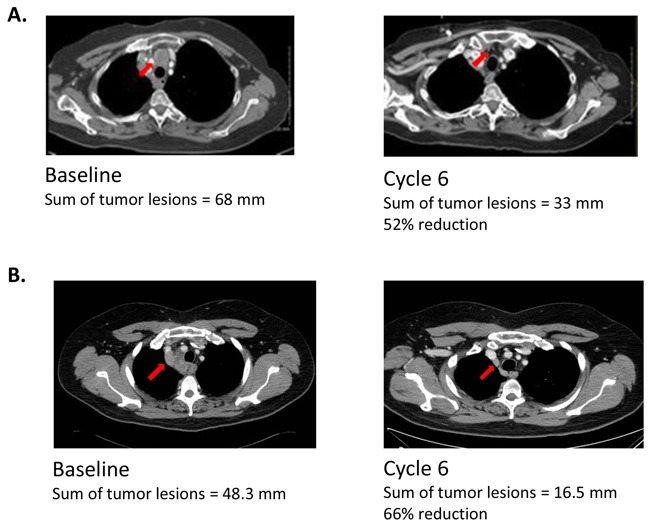
Tumors indicated by arrows. (A) Subject 001 (67-yr-old female) Subject 001 entered the study with extensive-stage disease after previously receiving six cycles of prior chemotherapy with carboplatin/etoposide over a 15-week period (total dose, carboplatin 3746 mg; etoposide 2964 mg). Her best response to prior therapy was SD of unknown duration. She also previously received two doses of palliative radiation therapy: 4500 rad to the right lung, and 3000 rad to the brain. After enrollment into the study, she received six cycles of study drug over an 18-week period, along with continuation of her last prior EP cycle (total dose, carboplatin, 660 mg, and etoposide, 420 mg) before beginning amuvatinib and had a best response of PR (119 days) in at least two assessments confirmed by independent radiologic review. (B) Subject 023 (52-yr-old male) Subject 023 entered the study with extensive-stage disease after previously receiving six cycles of cisplatin/etoposide over a 16-week period with a best response of SD of four months’ duration. He also previously received two doses of palliative radiation therapy: 7500 rad to the right hilar mass and mediastinal lymph nodes, and 2500 rad to the right supraclavicular area. After enrollment into the study, he received six cycles of study drug over a 19-week period along with continuation of his last prior EP cycle (total dose, cisplatin 127 mg, and etoposide, 765 mg) before beginning amuvatinib and had a PR (151 days) in at least two assessments confirmed by independent radiologic review.
