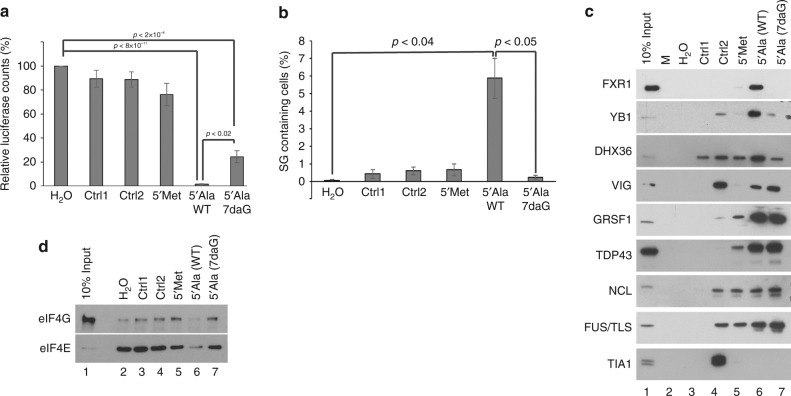
Fig. 5.
7-deazaguanine substitutions in 5ʹtiRNAAla prevent bioactivity. a In vitro translation assay in rabbit reticulocyte lysate using NanoLuc reporter to monitor translation efficiency. Substitution of two guanines in 5ʹtiRNAAla reduces its ability to repress translation. b Indicated RNAs were transfected into U2OS cells and stress granule formation was monitored through immunofluorescence. 5ʹtiRNAAla (WT) readily induces the formation of SGs, while 5ʹtiRNAAla(7daG) fails to. c RNA affinity purifications using indicated RNAs from U2OS lysates. Loss of RG4 forming ability in 5ʹtiRNAAla (7daG) correlates with loss of binding to YB-1, a protein required for tiRNA mediated SG formation. 5ʹtiRNAAla(7daG) also loses the ability to bind to Fxr1 and DHX36, previously identified RG4 binding proteins. In contrast, Vigilin, GRSF1, and TDP43 bind 5ʹtiRNAAla regardless of its ability to form RG4. d Inhibition of RG4 formation in 5ʹtiRNAAla through incorporation of 7-deazaguanine abrogates its ability to displace eIF4F from m7GTP agarose