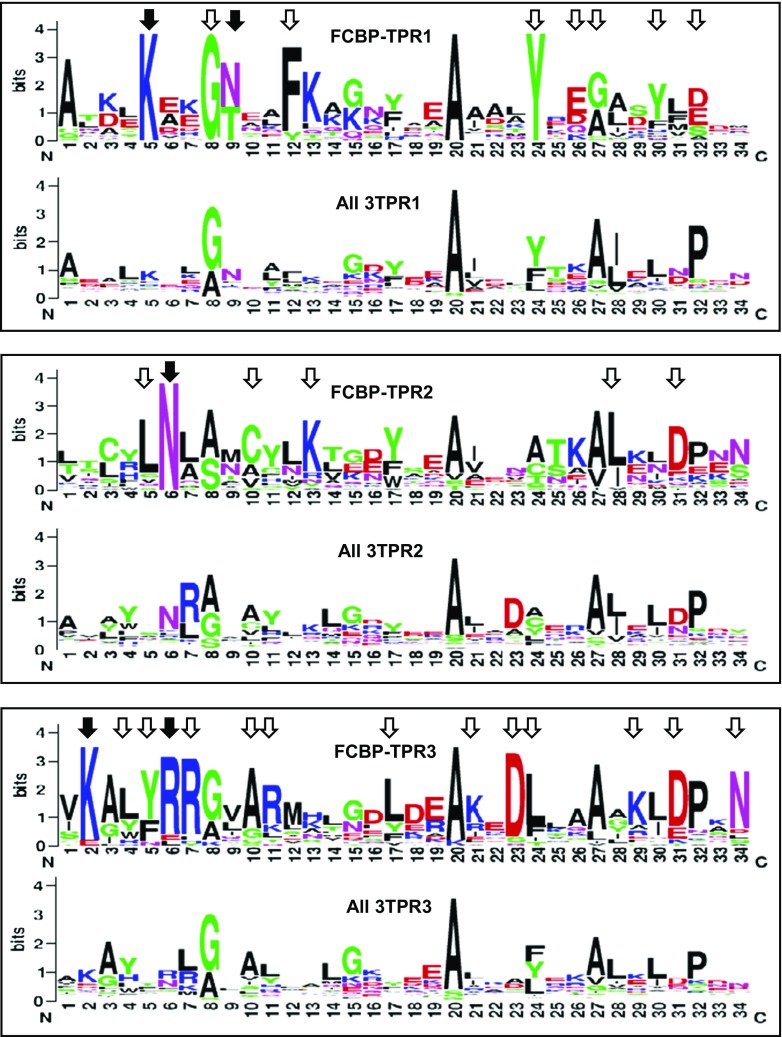
Fig. 5.
Sequence consensus among the three TPR motifs of FCBP. The 34-residue sequences of the TPR1, TPR2, and TPR3 motifs of all FCBPs were plotted in sequence logo format using the WebLogo software (Crooks et al. 2004). In each panel, the plot was compared with the corresponding TPR motif of all biological 3TPRs (Sawyer et al. 2013), as described in “Results.” Amino acid numbers (N- to C-terminus) are shown along the X-axis, and the Y-axis represents their relative abundance. The most conserved amino acid residues in the FCBP TPRs are indicated by all arrowheads. Residues that are known to interact with the MEEVD sequence of Hsp90, based on structural studies of CYN and FKBP TPRs (Cliff et al. 2006; Scheufler et al. 2000; Ward et al. 2002; Wu et al. 2004), are specifically indicated with closed arrowheads (detailed in Results); note that they are also among the most highly conserved, supporting the functional importance of a potential FCBP TPR-Hsp90 interaction