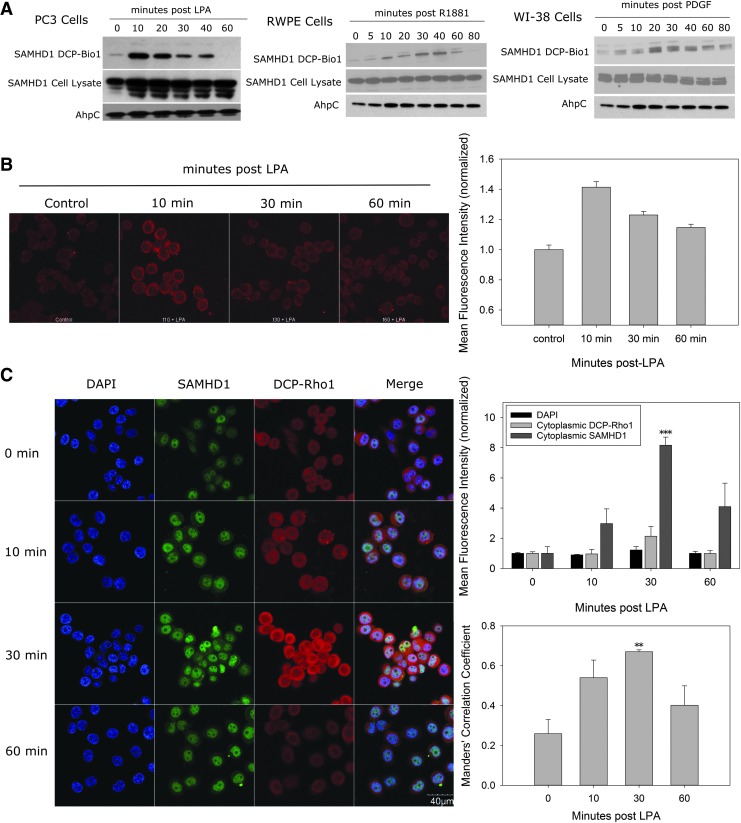
FIG. 5.
SAMHD1 is oxidized in cells in response to growth stimuli. (A) Oxidized proteins in PC3, RWPE, and WI-38 cells stimulated with LPA, R1881, and PDGF, respectively, were labeled with DCP-Bio1 and isolated with streptavidin beads. Captured protein was resolved by SDS-PAGE, blotted onto nitrocellulose, and probed with α-SAMHD1 antibodies. SAMHD1 oxidation increases poststimulation. Oxidized SAMHD1 and total SAMHD1 in the lysate are indicated. AhpC was used as a procedural control. Uncropped images of gels shown in Supplementary Figure S10. (B) Molecular colocalization of SAMHD1 and protein oxidation was visualized using a proximity ligation assay as described. The oxidized species of SAMHD1 (red signal) are present outside the nucleus. (C) On stimulation with LPA, total SAMHD1, imaged by immunofluorescence, moves outside the nucleus over time and colocalizes with areas of oxidized proteins labeled by DCP-Rho1. The mean fluorescence intensity of DAPI, cytoplasmic SAMHD1, and DCP-Rho1 was measured in about 100 cells at each time point, showing an increase in cytoplasmic SAMHD1. Manders' correlation coefficient representing the fraction of total cytoplasmic DCP-Rho1 that colocalizes with pixels containing SAMHD1 signal indicates an increasing colocalization of SAMHD1 with DCP-Rho1. Error bars represent standard error of the mean (**p < 0.01, ***p < 0.001). DAPI, 4′,6-diamidino-2-phenylindole; DCP-Bio1, dimedone coupled probe to biotin; DCP-Rho1, dimedone coupled probe to rhodamine; LPA, lysophosphatidic acid; PDGF, platelet-derived growth factor.