Abstract
Regardless of the cause of organ fibrosis, its main unwanted consequence is the formation of collagen fibril-rich deposits that hamper the structure and function of affected tissues. Although many strategies have been proposed for the treatment of fibrotic diseases, no therapy has been developed, which can effectively block the formation of collagen fibril deposits. With this in mind, we recently developed an antibody-based therapy to block key interactions that drive collagen molecules into fibrils. In this study, we analyzed target specificity, which is a main parameter that defines the safe use of all antibody-based therapies in humans. We hypothesized that, regardless of the route of administration, our antibody would preferentially bind to free collagen molecules synthesized at the sites of fibrosis and have minimal off-target interactions when applied in various tissues. To test this hypothesis, we used two experimental models of organ fibrosis: (1) a keloid model, in which antibody constructs were directly implanted under the skin of nude mice and (2) an experimental model of pulmonary fibrosis, in which our antibody was administered systemically by intravenous injection. Following administration, we studied the distribution of our antibody within target and off-target sites as well as analyzed its effects on fibrotic tissue formation. We found that local and systemic application of our antibody had high specificity for targeting collagen fibrillogenesis and also appeared safe and therapeutically effective. In summary, this study provides the basis for further testing our antifibrotic antibody in a broad range of disease conditions and suggests that this treatment approach will be effective if delivered by local or systemic administration.
Keywords: : fibrosis, therapeutic antibody, pulmonary fibrosis, keloid, collagen, fibrillogenesis
Introduction
Fibrosis is a serious clinical problem that affects various tissues and organs, including skin, lung, liver, kidney, cornea, joint capsule, fascia, and others.(1) A common feature of organ fibrosis is the accumulation of deposits that damage the structure and alter the function of involved tissues. These deposits consist primarily of collagen fibrils that form a resilient structure stabilized by the presence of chemical cross-links.
Given that collagen deposits are a universal feature of all fibrotic tissues, it has long been speculated that treatments which block their formation might be effective in ameliorating pathological tissue scarring.(2) Since collagen I comprises almost the entirety of established tissue scars, scientists have gone to great lengths to block various steps in collagen I biosynthesis, including at the gene transcription level and at various levels of intracellular posttranslational modification.(3,4) However, despite demonstrating efficacy in cell culture systems, the feasibility of applying many of these approaches in human disease remains unclear.
In addition to aforementioned approaches, scientists have also targeted extracellular steps of collagen modifications, including where procollagen N proteinase (PNP) and procollagen C proteinase (PCP) cleave propeptides of collagen to expose the telopeptides triggering collagen fibril formation.(5,6) Several inhibitors of PCP, including acidic dipeptide hydroxamate, have been applied to block PCP in vitro, but PCP knockout experiments have demonstrated that normal collagen fibrils still exist in various tissues, indicating that PCP alone is not responsible for processing procollagen in vivo.(7,8) Similarly, PNP knockout experiments have also demonstrated that fully processed collagen I and collagen III can be found in PNP-deficient tissues, indicating that therapeutic approaches targeting this enzyme are unlikely to be highly effective.(9)
Finally, because extracellular cross-linking of collagen molecules stabilizes the collagen fibrils, scientists have recently tested whether fibrotic deposits could be reduced by blocking the enzymes that catalyze the cross-link formation. Initially, it was determined that blocking lysyl oxidase (LOX) activity reduced pulmonary fibrosis in animal models.(10–13) However, subsequent clinical trials using an anti-LOX2 antibody, also known as simtuzumab, were terminated early due to lack of efficacy in idiopathic pulmonary fibrosis.(14) These results, and the approaches mentioned above, illustrate that targeting the intracellular and extracellular processing of collagen molecules remains a challenging problem.
In hopes of developing a novel therapy for organ fibrosis, our group postulated that directly inhibiting the self-assembly of collagen molecules into fibrils might be a more effective approach for reducing pathological organ fibrosis.(2) Specifically, our strategy was to block the assembly of individual collagen molecules into newly formed fibrils, knowing that this interaction depends on site-specific binding between the telopeptide region of one collagen molecule and the telopeptide binding region of an interacting partner.(15) To this end, we developed a recombinant antibody that specifically binds to the C-terminal telopeptide of the α2 chain (α2Ct) of collagen I.(2,16,17) Furthermore, we demonstrated that this recombinant antibody, referred to as the antifibrotic antibody (AFA), has antifibrotic properties in various in vitro and in vivo model systems.(2,16,18–20) For example, we showed the ability of our AFA to limit fibrosis in a rabbit-model of posttraumatic arthrofibrosis.(20)
To date, our studies suggest that treatment with AFA is safe and that our antibody binds to its intended target. For instance, this antibody lacked any cell toxicity when applied to a number of primary cells in culture.(16,17) Moreover, we demonstrated that our AFA binds specifically to individual collagen molecules before they are incorporated into fibrils in vitro. While our published in vivo studies in mice and in rabbits also suggest our AFA has no unwanted side effects, we have yet to determine whether our AFA has significant off-target binding in vivo.(2,21) Our present study analyzed potential off-target binding of the AFA in mouse models of keloid and pulmonary fibrosis. Our findings provide a basis for further preclinical testing of our AFA to determine its efficacy in limiting the consequences of organ fibrosis in a wide range of tissues and in vivo model systems.
Materials and Methods
Production of the AFA
We used CHO cells to produce the recombinant human IgG form of the AFA, as we have described elsewhere.(17,20) The antibody was isolated from cell culture media, concentrated, and then sterilized according to methods we described.(2,17,20)
Keloid-like constructs
All mice received humane care according to the guidelines in the Guide for the Care and Use of Laboratory Animals. Procedures performed on animals were approved by the Thomas Jefferson University's Institutional Animal Care and Use Committee.
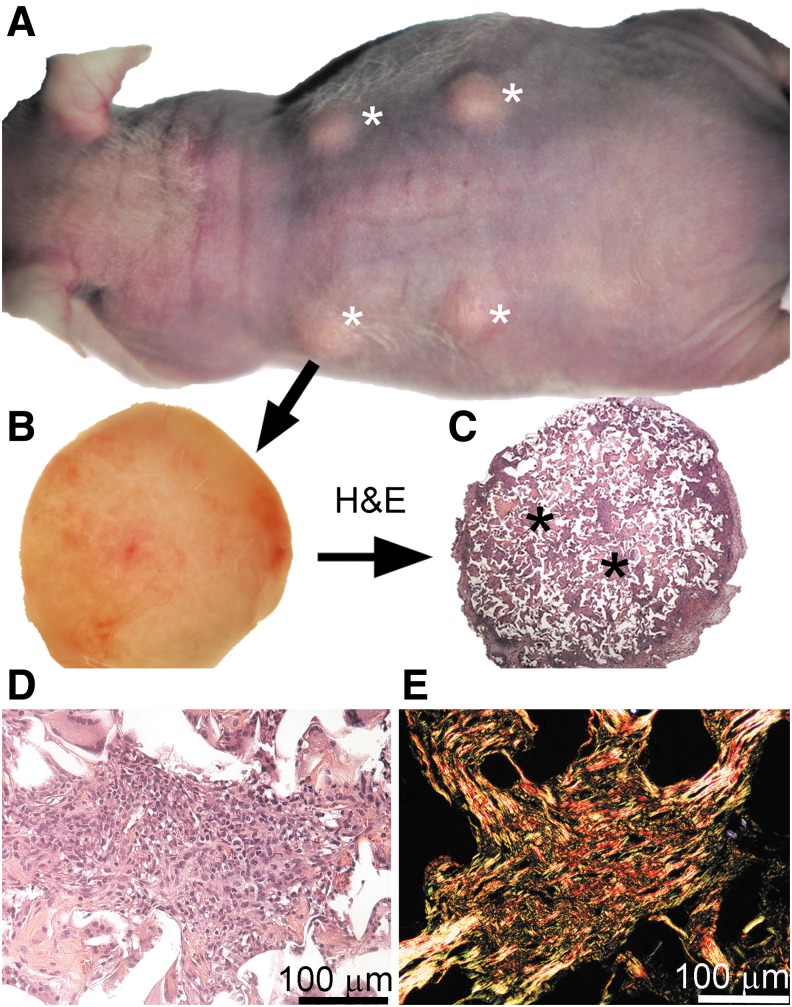
We used keloid-like constructs prepared according to our earlier protocols (Fig. 1).(2) In brief, we generated the organotypic constructs using three-dimensional open-cell poly-lactic acid scaffolds (OPLA; BD Biosciences). Keloid-derived fibroblasts were seeded dynamically into the scaffolds using a bioreactor (Sythecon, Inc., Houston, TX). Subsequently, these constructs were implanted subcutaneously into nude mice (Nude-Foxn1nu; HARLAN Laboratories, Inc., Indianapolis, IN). Next, those constructs received a 100-μL dose of the AFA present at a concentration of 1.5 mg/mL; similar doses of the AFA were injected every 3 days directly into the scaffolds and into surrounding areas. After 1 month, the mice were sacrificed, and the scaffolds were prepared for biochemical and morphological analyses, as described.(2) In parallel experiments, control mice received nonspecific human IgG (hIgG, Thermo Fisher Scientific, Waltham, MA). For each group, 4 keloid-like constructs were implanted under the skin of mice.
FIG. 1.
A keloid-like model of subcutaneous skin fibrosis. (A) Nude mouse with four subcutaneous keloid-like inserts (asterisks). (B) Keloid construct collected from a mouse. (C) H&E staining of the cross section of the construct seen in (B). (D) H&E staining of cells present in the cavities of the keloid-like constructs. (E) Picrosirius red-stained collagen fibrils deposited within cavities of the keloid-like constructs. (D, E) Present cavities from the central region of a scaffold, similar to that in (C) (asterisk). Scale bars = 100 μm.
Systemic delivery of the AFA
Before applying the AFA in an organ fibrosis model, we analyzed the time-dependent concentration of this antibody in the bloodstream following intravenous (i.v.) injection. In brief, n = 5 healthy mice received a single injection of this antibody at 30 mg/kg. A separate group of mice, n = 3, received the same amount of control hIgG. Subsequently, we analyzed antibody concentration in plasma at selected time points with the use of the human IgG-specific ELISA kit (Abcam, Cambridge, MA). Each sample was diluted to three different concentrations, each prepared in duplicate. Subsequently, the amount of IgG was calculated based on the standard curve prepared according to a manufacturer's protocol.
Lung fibrosis model
We used a model of bleomycin (BL)-induced pulmonary fibrosis. Specifically, we generated acute lung injury with a single intratracheal dose of BL. This model aims to recapitulate the fibrotic remodeling that develops in response to the acute pulmonary insult caused, for example, by inhalation of toxic gases, severe pulmonary or systemic infections, or by inhalation of massive quantities of organic or inorganic dust particles, but is also used as a model of idiopathic pulmonary fibrosis, the most common chronic fibrotic lung disease in older adults.
Two days after BL treatment, the mice received 20 mg/kg of the AFA via i.v. injection. The injection of the AFA was repeated every 3 days. Subsequently, the mice were sacrificed 18 days after BL injury. In parallel experiments a group of control mice was treated with nonspecific hIgG.
Collection of keloid-like constructs and assays of collagen content
Following the sacrifice of mice, the keloid-like constructs were collected. Each construct was divided into two samples; one of them was used for microscopy while the other one for chemical assays of collagen content.(2) After determining the concentration of collagen-specific hydroxyproline, the collagen content was calculated and then normalized with respect to DNA present within the keloid-like constructs, as described.(22)
Values for the collagen content in individual scaffolds were then used to calculate the means. Next, we reported individual data points and the means with 95% confidence intervals (CIs) for the AFA-treated and hIgG-treated groups (GraphPad Prism v. 5.03, GraphPad Software, Inc., La Jolla, CA).
Microscopic assays of the distribution of the AFA in mice harboring keloid-like constructs
In addition to harvesting the scaffolds, selected tissues and organs were also collected for immunohistology-based assays to determine the tissue distribution of applied antibodies. For these assays, we used the VECTASTAIN Elite ABC kit that includes biotinylated anti-human IgG antibody, avidin, and biotinylated horseradish peroxidase (HRP; Vector Laboratories, Inc., Burlingame, CA). Utilizing this kit, tissue-located AFA or control hIgG were ultimately detected using Vector NovaRED HRP substrate.
In all assays, histological specimens were counterstained with Methyl Green (Vector Laboratories, Inc.). Moreover, negative controls, in which the biotinylated anti-human IgG antibody was omitted, were also prepared.
Quantitative microscopy of collagen fibrils in lungs
In the lung fibrosis model, mice were sacrificed and the lungs were inflated with 4% paraformaldehyde. Subsequently, the lungs were processed for histology. In addition to the lungs, selected tissues and organs were also collected for immunohistology to detect the AFA and hIgG, as described above.
All collagen fibrils present in keloid-like constructs were formed de novo by the cells seeded into scaffolds. In contrast, in addition to collagen fibrils formed de novo in the BL-treated lungs, native collagen fibrils were also present in blood vessels and airways. The presence of these native collagen fibrils causes a problem for chemical measurements of novel collagen deposits formed in the lung tissue.(23) To circumvent this problem, instead of using the hydroxyproline-based assays of the collagen content in lungs, we used quantitative microscopy. This method allows direct measurement of the collagen fibrils present only in well-defined fibrotic lesions. For these direct assays, we used picrosirius red-stained samples according to described protocols.(20,21) In brief, picrosirius-stained sections were photographed first in normal light and then in polarized light (Eclipse LV100POL; Nikon, Inc., Melville, NY). For assays of the density of fibrils, the regions of interest (ROIs), corresponding to the areas of the lesions, were delineated in the normal-light images. Then, these ROIs were automatically copied to the corresponding areas of images taken in polarized light. Subsequently, the fibrils within the ROIs were automatically detected by the NIS-Elements software (Nikon, Inc.). Finally, we calculated the total area of each ROI and the percent area occupied by fibrils within each ROI.
For quantitative microscopy assays, we used n = 5 BL-injured AFA-treated mice, n = 7 BL-injured hIgG-treated mice, and n = 3 BL-injured nontreated mice. On average, we analyzed five ROIs per mouse. Subsequently, we calculated the mean percent area for each analyzed group. Next, we compared the statistical significance of the differences between the means of the following group pairs: (1) BL-treated mice that received the AFA and BL-treated mice that received hIgG, (2) BL-treated mice that received the AFA and BL-treated mice that did not receive any antibody, and (3) BL-treated mice that received hIgG and BL-treated mice that did not receive any antibody. The Student's t-test was used to determine the statistical significance of these differences (GraphPad Prism v. 5.03, GraphPad Software, Inc.). We also plotted graphs relating the density of collagen fibrils to the size of lesions defined as the ROI.
Picrosirius red staining of selected tissues and organs
In addition to immunohistology to detect the AFA or hIgG, collected specimens were stained with picrosirius red to visualize collagen fibrils. The picrosirius red-stained samples were observed under polarized light.
Results
Distribution of the AFA after local administration in a keloid-like model
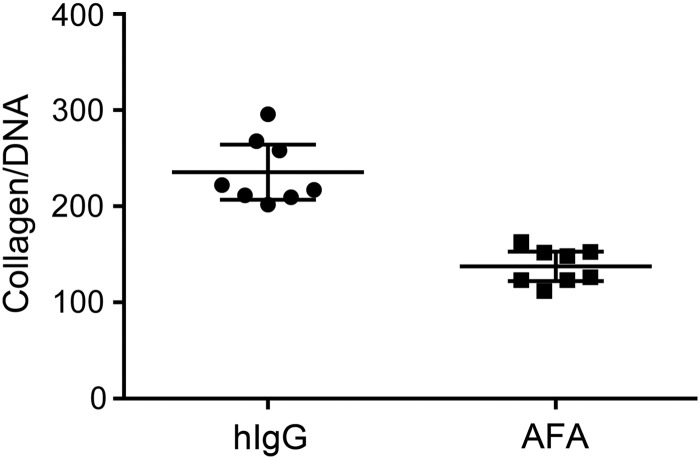
Our earlier studies demonstrated that the anti-α2Ct antibody significantly reduces collagen accumulation in the cavities of subcutaneous keloid-like constructs.(2) As expected, results presented in this study show a similar trend, thereby validating the used experimental system (Fig. 2).
FIG. 2.
Graphic representation of the collagen content in the keloid-like constructs treated with the AFA or hIgG. The amount of collagen content is expressed with respect to a unit mass of DNA. The individual data points and the means with 95% CI are presented. AFA, antifibrotic antibody; CI, confidence interval.
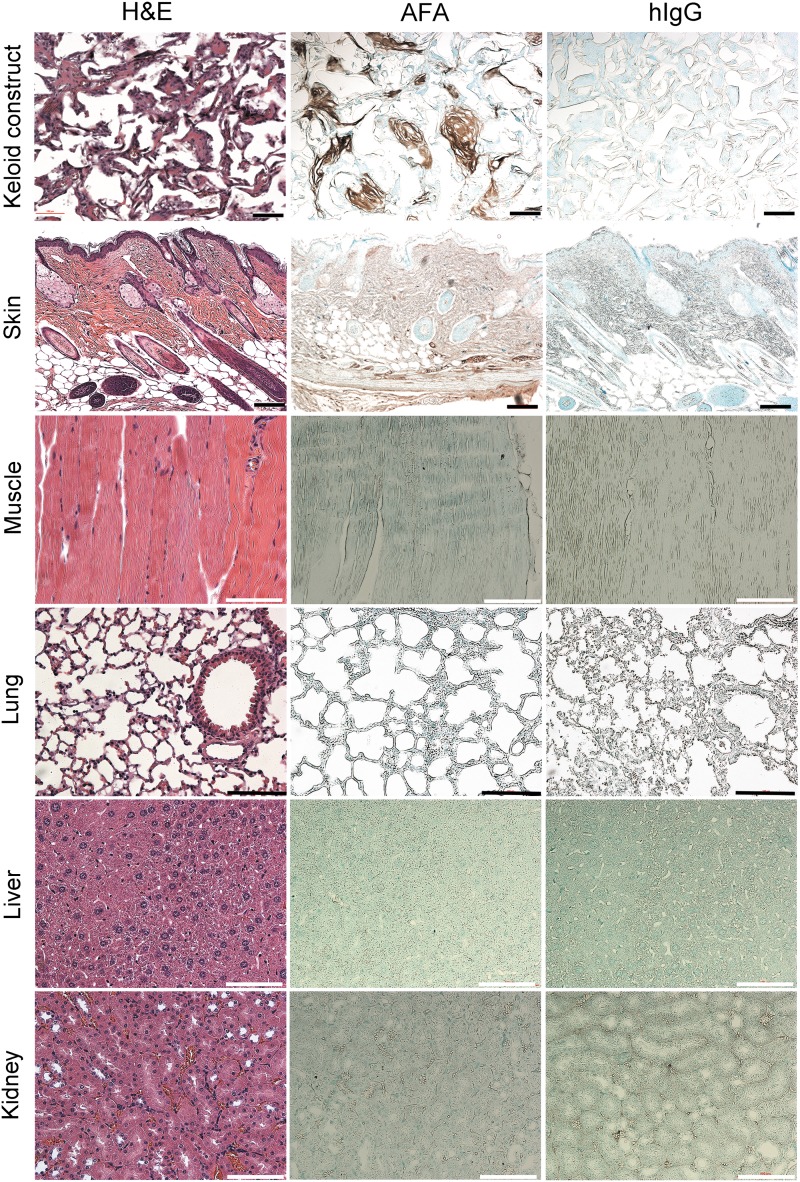
In this study, we focused on determining off-target effects of our AFA by analyzing its cross-reactivity within tissues juxtaposed to collagenous matrices. Immunohistology to detect the AFA demonstrated strong staining in the cavities of subcutaneous scaffolds (Fig. 3). In contrast, only a weak AFA-positive signal was detected in the dermis collected from the area where we injected the antibody. Moreover, after injecting our antibody into subcutaneous keloid-like constructs, we did not observe any accumulation of the AFA in distant organs (Fig. 3). In contrast to the AFA-treated mice, samples from the hIgG-treated mice showed no readily detectable staining (Fig. 3). We confirmed the specificity of AFA detection by using assays that showed no staining in negative controls, in which only the secondary antibody was applied (data not shown).
FIG. 3.
Immunohistology of keloid-like constructs and selected tissues to detect potential accumulation of the AFA or hIgG. Note that readily visible staining is only observed in the AFA-treated keloid-like construct. Positive staining is absent in distant organs of both AFA-treated and hIgG-treated mice and is absent from keloid-like constructs when exposed to hIgG control antibody. Scale bars = 100 μm.
Systemic administration of the AFA in a lung fibrosis model
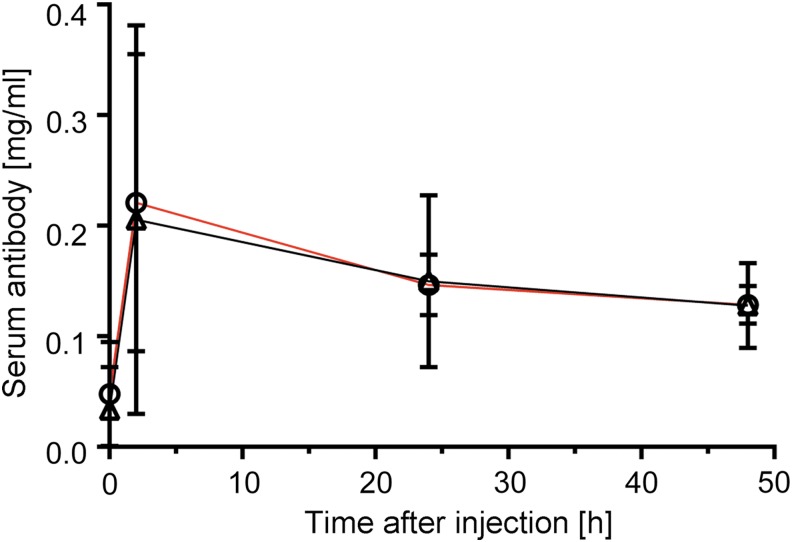
When the AFA was injected intravenously into healthy mice, about 65% of the initial amount persisted in the bloodstream 48 hours after injection, according to enzyme-linked immunosorbent assay. The AFA clearance from the bloodstream was kinetically similar to that that of the hIgG control (Fig. 4).
FIG. 4.
Plasma concentration of the AFA (black line) and hIgG (red line). The lines represent time-dependent changes in plasma antibody concentration after i.v. injection of 30 mg/kg into healthy mice.
Measurements of collagen fibrils in fibrotic lesions of the lungs
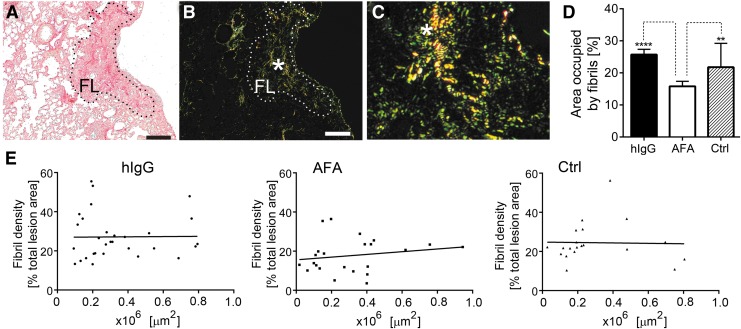
Quantitative histology of the picrosirius red-stained lungs demonstrated that the amount of collagen fibrils in the fibrotic lesions was significantly reduced in the presence of the AFA. In contrast, the amount of collagen fibrils present in mice treated with hIgG did not change significantly in comparison with nontreated mice with the BL-induced lung injury (Fig. 5). We also analyzed the relationship between the density of fibrils and the size of the area of the lesions to determine the reliability of the applied method. The graphs presented in Figure 5E show that, essentially, the lesion area did not have any significant effect on fibril density, thereby indicating a uniform sampling procedure across ROIs.
FIG. 5.
Quantitation of picrosirius red-stained collagen fibrils present in the ROIs corresponding to the fibrotic lesions of the lungs from BL-treated mice. (A, B) Representative images of a readily visible FL (delineated) observed in normal light (A) and polarized light (B). (C) High-magnification view of collagen fibrils seen in the corresponding region (asterisk) in (B). (D) Graphic representation of the area occupied by collagen fibrils present in the lesions of the hIgG-treated mice, AFA-treated mice, and nontreated mice (Ctrl). (E) A graphic illustration of the relationship between the size of lesions and the density of collagen fibrils. Asterisks indicate statistically significant differences between analyzed groups: **p < 0.01, ****p < 0.0001. Scale bars = 50 μm. BL, bleomycin; FL, fibrotic lesion; ROI, region of interest.
Distribution of the AFA after systemic administration in a lung fibrosis model
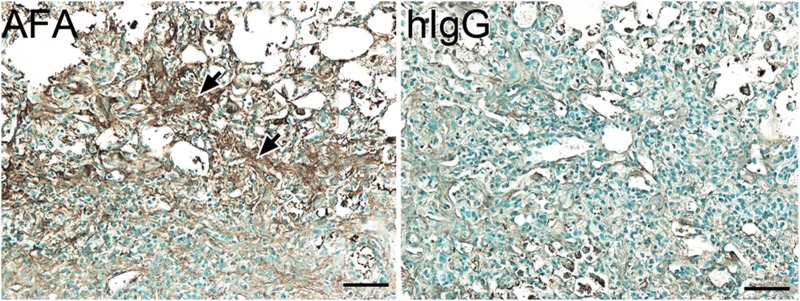
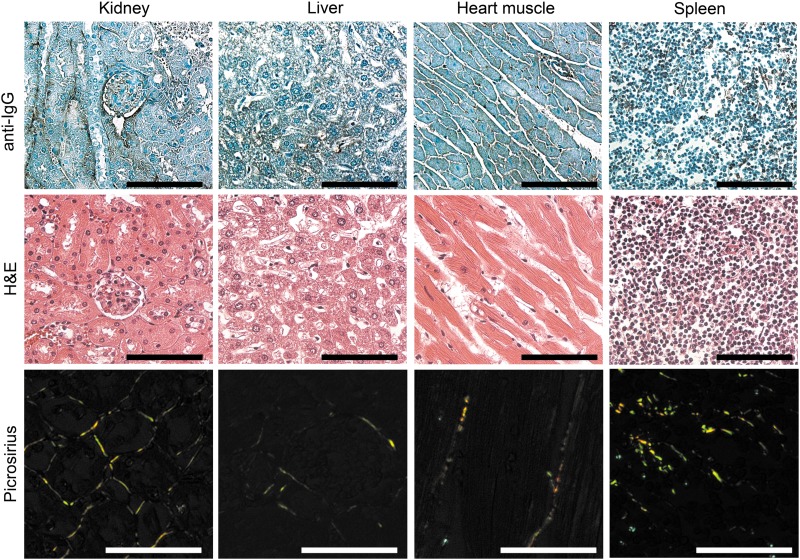
When we carried out immunohistological assays with tissue sections from injured lungs and uninjured organs to detect the accumulation of i.v.-applied antibodies, we found readily visible staining only in the BL-injured lungs of the AFA-treated mice (Figs. 6 and 7). Similar staining was largely absent in the corresponding group of mice treated with control hIgG (Fig. 6), and no staining was visible in negative control groups of histological samples, in which the biotinylated anti-human IgG antibody was not applied (not shown). Picrosirius red staining of uninjured organs visualized the presence of the collagen fibrils that form the extracellular scaffolds of these organs. Despite their presence, no AFA accumulation was observed. These results confirmed the target specificity of the applied staining method.
FIG. 6.
Immunohistology assays of exogenous IgG in the lungs of the BL-treated mice that received the AFA or hIgG. The readily visible signal from the deposited antibody is only seen in the fibrotic lesions of AFA-treated mice (arrows). Scale bars = 50 μm.
FIG. 7.
Immunohistology assays of exogenous IgG in selected tissues of the BL-treated mice that received the AFA. Corresponding areas stained with H&E or picrosirius red are also shown. Picrosirius red-stained samples were observed under a polarizing microscope to visualize the presence of various collagenous structures. Scale bars = 50 μm.
Discussion
Fibrotic diseases commonly feature the accumulation of disorganized collagen-rich deposits that alter the structure and function of affected tissues. Our group focused on limiting the deposition of collagen fibrils formed in response to tissue injury by targeting collagen fibrillogenesis. Recently, we validated this target in vivo in a rabbit model of posttraumatic arthrofibrosis,(20) demonstrating that blocking collagen fibrillogenesis with our AFA reduced collagen fibril accumulation and thereby improved joint motion.(20)
Because our group has already demonstrated the validity of targeting collagen fibrillogenesis in a number of studies, the primary objective of this study was to verify in vivo target specificity of our antifibrotic approach before moving onto larger preclinical testing in various animal models.(2,16,17,20)
Antibody-based therapeutics are generally highly specific, but off-target binding can sometimes present a significant concern.(24,25) Because of this issue, the Food and Drug Administration (FDA) recommends analyzing the specificity of all antibodies as a critical parameter in defining its safety.(26) One method for testing antibody specificity is to examine its reactivity within a wide range of tissues. By performing these types of analyses, we have now verified that our AFA has virtually no interactions with tissues that do not include our collagen I target.(17)
Our earlier AFA cross-reactivity studies have some limitations. For instance, we do not know from these studies if relevant epitopes in used arrays of tissues were intact and accessible for interactions with the AFA. We also cannot exclude the possibility that some epitopes, fully masked in their native form, were partially exposed during tissue array preparation. Based on these considerations, we cannot exclude some false-negative or false-positive results obtained with the tissue arrays-based assays of the AFA cross-reactivity.(17)
The potential cross-reactivity of the AFA in vivo is a valid initial concern due to ubiquitous presence of collagen I-based fibrils in the body. Our study presented here, however, demonstrated a lack of binding of this antibody to established fibrils. Regardless of the route of administration, the AFA was only detected in areas with active production of collagen I monomers. In the keloid model, the AFA was primarily detected in cavities of the subcutaneous scaffolds and staining was only visible at sites of lung injury in our pulmonary fibrosis model. The observation that AFA treatment significantly reduced collagen-rich deposits in both models not only corroborates the efficacy of our antibody in vivo but also further documents its target specificity.
Active production of collagen I molecules is a common characteristic of the target sites in keloid and lung fibrosis models. In the keloid model, this production results from inherent properties of keloid-derived fibroblasts to excessively produce collagen I molecules that assemble into fibrils, while in the lung fibrosis model, collagen I molecules result from a fibrotic response to the BL-mediated lung injury.
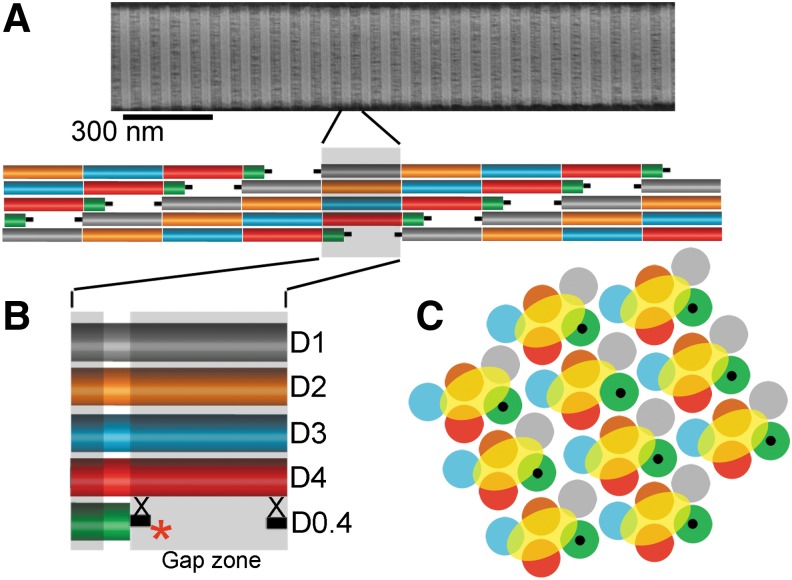
The absence of AFA-specific immunostaining of uninjured organs and tissues indicates that the AFA does not accumulate in sites with existing mature collagen I-rich fibrils. This result supports our earlier observation that the accessibility of the α2Ct epitope in mature fibrils is limited.(16) This was confirmed by using an electron microscopy to analyze the interaction of the AFA with established fibrils formed in vitro by de novo self-assembly of collagen I molecules. The electron microscopy analysis showed a paucity of AFA molecules bound to these fibrils, and those AFA molecules appeared only in the gap regions of collagen fibrils, in which the α2Ct target sites are present (Fig. 8).(16) We expect that the accessibility of the AFA to the α2Ct target sites is even more limited in native fibrils than in those formed in vitro. Specifically, in contrast to the homotypic fibrils formed in vitro by assembly of collagen I molecules, the native collagen fibrils formed in vivo are heterotypic due to coassembly with collagen III and collagen V.(27,28) Moreover, proteoglycans and fibril-associated collagen types frequently decorate the surface of native collagen fibrils, which limits the binding of other molecules, including the AFA. Because of this characteristic, Sweeney et al. suggested that the gap zone serves as a “matrix interaction domain” to which matrix molecules bind and connect the fibril with other extracellular matrix elements.(29)
FIG. 8.
A schematic of the α2Ct region recognized by the AFA. The α2Ct region is presented in the context of collagen molecules that form a fibril. (A) Collagen fibrils observed in an electron microscope. (B) Simplified model of a collagen microfibril, with each collagen molecule depicted to show specific regions that correspond to the D-periods. Each D-period is indicated by unique color. Collagen telopeptides, indicated as black bars, flank each collagen molecule. A gap zone with the AFA (asterisk) bound to the α2Ct region is also shown. The position of cross-links is also indicated (X). (C) Preserving colors of specific D-periods, the arrangement of multiple microfibrils, each highlighted by a yellow oval, in a cross section of a fibril (based on Hulmes et al.(30)). This arrangement depicts masking of the α2Ct region, (indicated in black) present at the C terminus of the D0.4 period (green), by interacting collagen molecules.
These considerations may explain why the AFA rarely binds to established fibrils in vivo. Other possible reason, based on our research, may be due to steric hindrance imposed by the overlap of collagen molecules that form a fibril. Moreover, we cannot exclude the possibility that cross-links in native fibrils in regions adjacent to the α2Ct epitope may further hamper AFA binding to the established fibrillar matrices (Fig. 8).
Our research shows that the AFA preferentially binds to the individual collagen I molecules rather than to mature fibrils, indicating that this antibody targets sites of active fibrillogenesis. Because of this characteristic of the AFA, the technology we propose to reduce the production of collagen-rich deposits formed in response to tissue injury opens new, site-specific approaches to develop effective and safe therapies to limit fibrosis. Ongoing research aims to determine the effects of this technology on processes of biosynthesis and degradation of collagen-rich matrices that are a natural part of the tissue turnover that is constantly taking place in physiological conditions, which will be important before advancing this technology to the clinic.
Acknowledgments
This work was supported by a grant from NIH to A.F. (AR048544). The authors thank Jennifer Fisher Wilson for revising the article.
Author Disclosure Statement
The authors have declared that no competing interest exists. A.F. has applied for the protection of the sequence of the antibody used in this study.
References
- 1.White ES, and Mantovani AR: Inflammation, wound repair, and fibrosis: Reassessing the spectrum of tissue injury and resolution. J Pathol 2013;229:141–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung HJ, Steplewski A, Chung KY, Uitto J, and Fertala A: Collagen fibril formation. A new target to limit fibrosis. J Biol Chem 2008;283:25879–25886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bateman ED, Turner-Warwick M, and Adelmann-Grill BC: Immunohistochemical study of collagen types in human foetal lung and fibrotic lung disease. Thorax 1981;36:645–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kagan HM: Intra- and extracellular enzymes of collagen biosynthesis as biological and chemical targets in the control of fibrosis. Acta Trop 2000;77:147–152 [DOI] [PubMed] [Google Scholar]
- 5.Fertala A, Holmes DF, Kadler KE, Sieron AL, and Prockop DJ: Assembly in vitro of thin and thick fibrils of collagen II from recombinant procollagen II. The monomers in the tips of thick fibrils have the opposite orientation from monomers in the growing tips of collagen I fibrils. J Biol Chem 1996;271:14864–14869 [DOI] [PubMed] [Google Scholar]
- 6.Kadler KE, Holmes DF, Trotter JA, and Chapman JA: Collagen fibril formation. Biochem J 1996;316 (Pt 1):1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovens A, Joule JA, and Kadler KE: Design and synthesis of acidic dipeptide hydroxamate inhibitors of procollagen C-proteinase. J Pept Sci 2000;6:489–495 [DOI] [PubMed] [Google Scholar]
- 8.Suzuki N, Labosky PA, Furuta Y, Hargett L, Dunn R, Fogo AB, Takahara K, Peters DM, Greenspan DS, and Hogan BL: Failure of ventral body wall closure in mouse embryos lacking a procollagen C-proteinase encoded by Bmp1, a mammalian gene related to Drosophila tolloid. Development 1996;122:3587–3595 [DOI] [PubMed] [Google Scholar]
- 9.Li SW, Arita M, Fertala A, Bao Y, Kopen GC, Langsjo TK, Hyttinen MM, Helminen HJ, and Prockop DJ: Transgenic mice with inactive alleles for procollagen N-proteinase (ADAMTS-2) develop fragile skin and male sterility. Biochem J 2001;355:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barry-Hamilton V, Spangler R, Marshall D, McCauley S, Rodriguez HM, Oyasu M, Mikels A, Vaysberg M, Ghermazien H, Wai C, Garcia CA, Velayo AC, Jorgensen B, Biermann D, Tsai D, Green J, Zaffryar-Eilot S, Holzer A, Ogg S, Thai D, Neufeld G, Van Vlasselaer P, and Smith V: Allosteric inhibition of lysyl oxidase-like-2 impedes the development of a pathologic microenvironment. Nat Med 2010;16:1009–1017 [DOI] [PubMed] [Google Scholar]
- 11.Cox TR, Bird D, Baker AM, Barker HE, Ho MW, Lang G, and Erler JT: LOX-mediated collagen crosslinking is responsible for fibrosis-enhanced metastasis. Cancer Res 2013;73:1721–1732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez HM, Vaysberg M, Mikels A, McCauley S, Velayo AC, Garcia C, and Smith V: Modulation of lysyl oxidase-like 2 enzymatic activity by an allosteric antibody inhibitor. J Biol Chem 2010;285:20964–20974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikenaga N, Peng ZW, Vaid KA, Liu SB, Yoshida S, Sverdlov DY, Mikels-Vigdal A, Smith V, Schuppan D, and Popov YV: Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut 2017;66:1697–1708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.U.S. National Institutes of Health. Study to assess the efficacy and safety of simtuzumab (GS-6624) in adults with idiopathic pulmonary fibrosis (IPF). 2017. https://clinicaltrials.gov/ct2/show/NCT01769196 Accessed September25, 2017
- 15.Prockop DJ, and Fertala A: Inhibition of the self-assembly of collagen I into fibrils with synthetic peptides. Demonstration that assembly is driven by specific binding sites on the monomers. J Biol Chem 1998;273:15598–15604 [DOI] [PubMed] [Google Scholar]
- 16.Fertala J, Kostas J, Hou C, Steplewski A, Beredjiklian P, Abboud J, Arnold WV, Williams G, and Fertala A: Testing the anti-fibrotic potential of the single-chain Fv antibody against the alpha2 C-terminal telopeptide of collagen I. Connect Tissue Res 2014;55:115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fertala J, Steplewski A, Kostas J, Beredjiklian P, Williams G, Arnold W, Abboud J, Bhardwaj A, Hou C, and Fertala A: Engineering and characterization of the chimeric antibody that targets the C-terminal telopeptide of the alpha2 chain of human collagen I: A next step in the quest to reduce localized fibrosis. Connect Tissue Res 2013;54:187–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arnold WV, and Fertala A: Skeletal diseases caused by mutations that affect collagen structure and function. Int J Biochem Cell Biol 2013;45:1556–1567 [DOI] [PubMed] [Google Scholar]
- 19.Steplewski A, and Fertala A: Inhibition of collagen fibril formation. Fibrogenesis Tissue Repair 2012;5 Suppl 1:S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steplewski A, Fertala J, Beredjiklian PK, Abboud JA, Wang MLY, Namdari S, Barlow J, Rivlin M, Arnold WV, Kostas J, Hou C, and Fertala A: Blocking collagen fibril formation in injured knees reduces flexion contracture in a rabbit model. J Orthop Res 2017;35:1038–1046 [DOI] [PubMed] [Google Scholar]
- 21.Steplewski A, Fertala J, Beredjiklian PK, Abboud JA, Wang ML, Namdari S, Barlow J, Rivlin M, Arnold WV, Kostas J, Hou C, and Fertala A: Auxiliary proteins that facilitate formation of collagen-rich deposits in the posterior knee capsule in a rabbit-based joint contracture model. J Orthop Res 2016;34:489–501 [DOI] [PubMed] [Google Scholar]
- 22.Jensen DA, Steplewski A, Gawron K, and Fertala A: Persistence of intracellular and extracellular changes after incompletely suppressing expression of the R789C (p.R989C) and R992C (p.R1192C) collagen II mutants. Hum Mutat 2011;32:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurent GJ: Lung collagen: More than scaffolding. Thorax 1986;41:418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Wang EQ, and Balthasar JP: Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2008;84:548–558 [DOI] [PubMed] [Google Scholar]
- 25.Pai R, Ma N, Connor AV, Danilenko DM, Tarrant JM, Salvail D, Wong L, Hartley DP, Misner D, Stefanich E, Wu Y, Chen Y, Wang H, and Dambach DM: Therapeutic antibody-induced vascular toxicity due to off-target activation of nitric oxide in cynomolgus monkeys. Toxicol Sci 2016;151:245–260 [DOI] [PubMed] [Google Scholar]
- 26.U.S. Food and Drug Administration. S6(R1) Preclinical safety evaluation of biotechnology-derived pharmaceuticals. 2017. https://www.fda.gov/drugs/guidancecomplianceregulatoryinformation/guidances/ucm304390.htm Accessed September25, 2017
- 27.Hansen U, and Bruckner P: Macromolecular specificity of collagen fibrillogenesis: Fibrils of collagens I and XI contain a heterotypic alloyed core and a collagen I sheath. J Biol Chem 2003;278:37352–37359 [DOI] [PubMed] [Google Scholar]
- 28.Woltersdorf C, Bonk M, Leitinger B, Huhtala M, Kapyla J, Heino J, Gil Girol C, Niland S, Eble JA, Bruckner P, Dreier R, and Hansen U: The binding capacity of alpha1beta1-, alpha2beta1- and alpha10beta1-integrins depends on non-collagenous surface macromolecules rather than the collagens in cartilage fibrils. Matrix Biol 2017 [DOI] [PubMed] [Google Scholar]
- 29.Sweeney SM, Orgel JP, Fertala A, McAuliffe JD, Turner KR, Di Lullo GA, Chen S, Antipova O, Perumal S, Ala-Kokko L, Forlino A, Cabral WA, Barnes AM, Marini JC, and San Antonio JD: Candidate cell and matrix interaction domains on the collagen fibril, the predominant protein of vertebrates. J Biol Chem 2008;283:21187–21197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hulmes DJ, Wess TJ, Prockop DJ, and Fratzl P: Radial packing, order, and disorder in collagen fibrils. Biophys J 1995;68:1661–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]