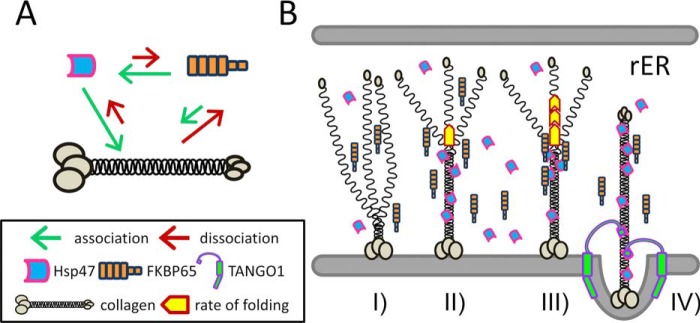
Figure 6.
Schematic diagram of the interactions between collagen, Hsp47, and FKBP65 and a proposed model of collagen folding in the rER. A, illustration of the interactions between three proteins; collagen, Hsp47, and FKBP65. The length of the arrows indicates the strength of the association (green) and dissociation (red) between molecules. B, graphical representation of the combined effects of Hsp47 and FKBP65 on collagen folding. I, triple helix formation is initiated from the carboxyl-terminal end; II, Hsp47 binds to the newly formed triple helix; III, FKBP65 is recruited to Hsp47 at the triple helix propagation site and accelerates the rate of folding; IV, FKBP65 is replaced by the SH3 domain of TANGO1 at the ER exit site for the loading of collagen into a special COPII vesicle.