Figure 4.
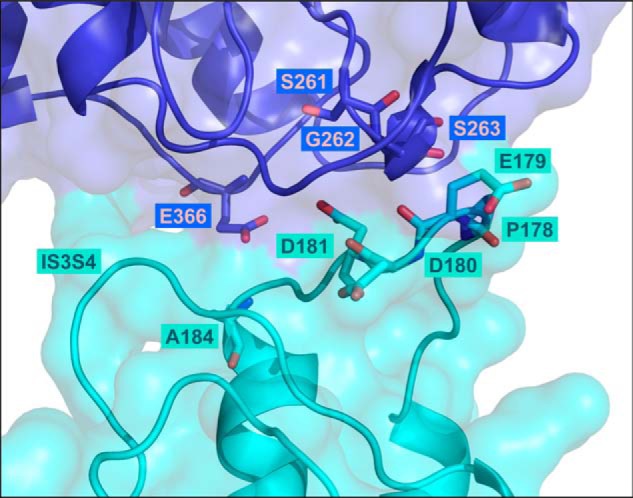
Three-dimensional model of the extracellular loops of the rabbit CaV1.2 in complex with the VWA domain of the rat CaVα2δ1 protein. The 3D model of the region spanning the first transmembrane helix S1 to the fourth transmembrane helix S4 in the first repeat in CaV1.2 (IS1S4) is shown in cyan, and the VWA domain of CaVα2δ1 is shown in deep blue. Residues Pro-178, Glu-179, Asp-180, Asp-181, and Ala-184 of CaV1.2 and residues Ser-261, Gly-262, Ser-263, and Glu-366 in CaVα2δ1 (three of the five residues in the MIDAS) are shown in stick representation with oxygen and nitrogen atoms colored in red and in blue, respectively. The model does not predict strong electrostatic interactions between CaV1.2 Asp-180 and residues in CaVα2δ1. Intramolecular interactions with residues in the extracellular IS3S4 loop are not ruled out. CaV1.2 Asp-181 appears to be appropriately oriented to form electrostatic interactions with Gly-262 and Ser-263 in CaVα2δ1. Modeling was achieved with Modeler 9.17. The figure was produced using PyMOL.
