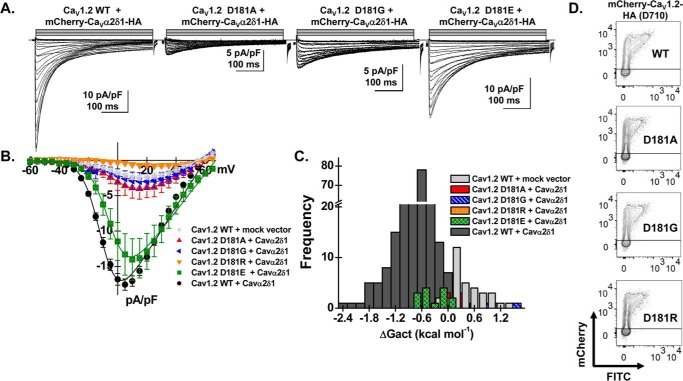
Figure 6.
Mutations at Asp-181 prevent up-regulation of CaV1.2 currents. HEKT cells were transiently transfected with pCMV–CaVβ3 and pmCherry–CaVα2δ1–HA with the CaV1.2 constructs (D181A, D181G, D181R, and D181E). A, whole-cell Ca2+ current traces were recorded in the presence of 2 mm Ca2+ from a holding potential of −100 mV for the constructs as identified. The current traces with the largest currents are shown for CaV1.2 constructs D181G and D181E. Time scale is 100 ms throughout. The current density scale is either 5 or 10 pA/pF as indicated. B, averaged current-voltage relationships. Peak current densities versus voltage relationships were measured for CaV1.2 WT and CaV1.2 mutants (as shown). Currents traces obtained with the empty mCherry (mock) vector are also shown. CaV1.2 constructs D181A, D181G, and D181R generated currents that were not significantly up-regulated by mCherry–CaVα2δ1–HA WT. Statistical analyses were performed with a one-way ANOVA test: *, p < 0.01, and **, p < 0.001 against the mock vector. See Table 1 for details. C, distribution of the free energies of activation. The values for the free energy of activation (ΔGact) measured for CaV1.2 constructs (D181A, D181G, D181E, and D181R) overlapped with the values measured for the mock vector. D, representative two-dimensional plots of mCherry versus FITC fluorescence. The cell-surface expression of the CaV1.2 mutants was evaluated by introducing the mutation in the mCherry–CaV1.2–HA construct. The surface fluorescence was estimated from the relative intensity of the fluorescence emitted by the fluorescein isothiocyanate (FITC)-conjugated anti-HA as measured using a flow cytometry assay (10,000 intact cells). The construct allows for detection of intracellular and extracellular fluorescence using FITC-conjugated anti-HA (“x axis”) and an anti-mCherry (“y axis”), respectively. The robust mCherry signal (y axis) confirms that the proteins were translated up to the end of the coding sequence. The cell-surface fluorescence for FITC, calculated as ΔMedFI as explained under “Experimental procedures,” was slightly lower for the CaV1.2 mutants (D181A, D181G, and D181R) than for the WT construct. Nonetheless, all constructs significantly fluoresced at the cell surface supporting the view that the absence of function did not result from a complete absence of trafficking to the cell membrane. Furthermore, the ΔMedFI signal for the total protein was similar for all WT and mutant constructs demonstrating that proteins were appropriately translated, an observation also obtained from carrying out routine Western blotting.