Figure 1.
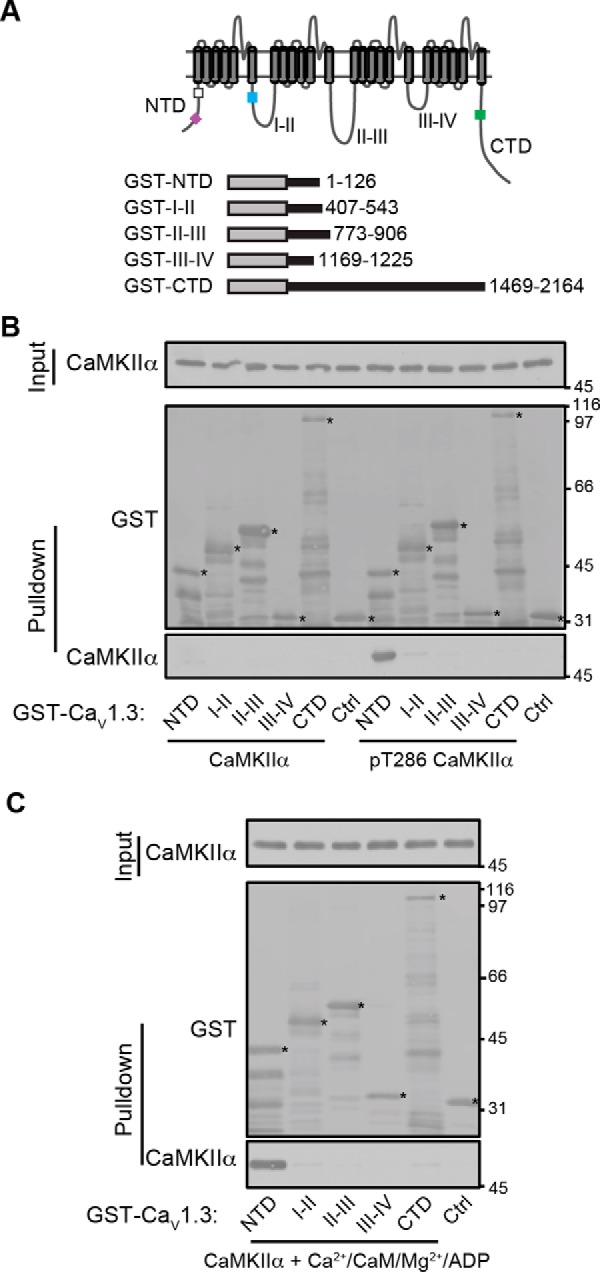
Activated CaMKII specifically binds to the LTCC NTD. A, domain structure of LTCCs and GST fusion proteins used here. Rectangular boxes in the intracellular domains indicate approximate positions of previously reported calmodulin (NSCaTE (24)) (purple box)-binding and CaMKII (20) (white box)-binding domains in the NTD, the α subunit interaction domain (AID, for β subunit interaction) in the I/II linker (50) (blue box), and overlapping calmodulin- and CaMKII-binding sites in the CTD (17) (green box). B, glutathione-agarose co-sedimentation assays show that there is no reliably detectable interaction of inactive (non-autophosphorylated) conformations of CaMKIIα with any of the CaV1.3 intracellular domains but that activated (pre-autophosphorylated) CaMKIIα specifically binds to the NTD. C, activation of CaMKIIα by binding of Ca2+/calmodulin and Mg-ADP is sufficient for interaction with the CaV1.3 NTD. The immunoblots shown are representative of three independent experiments.
