Figure 7.
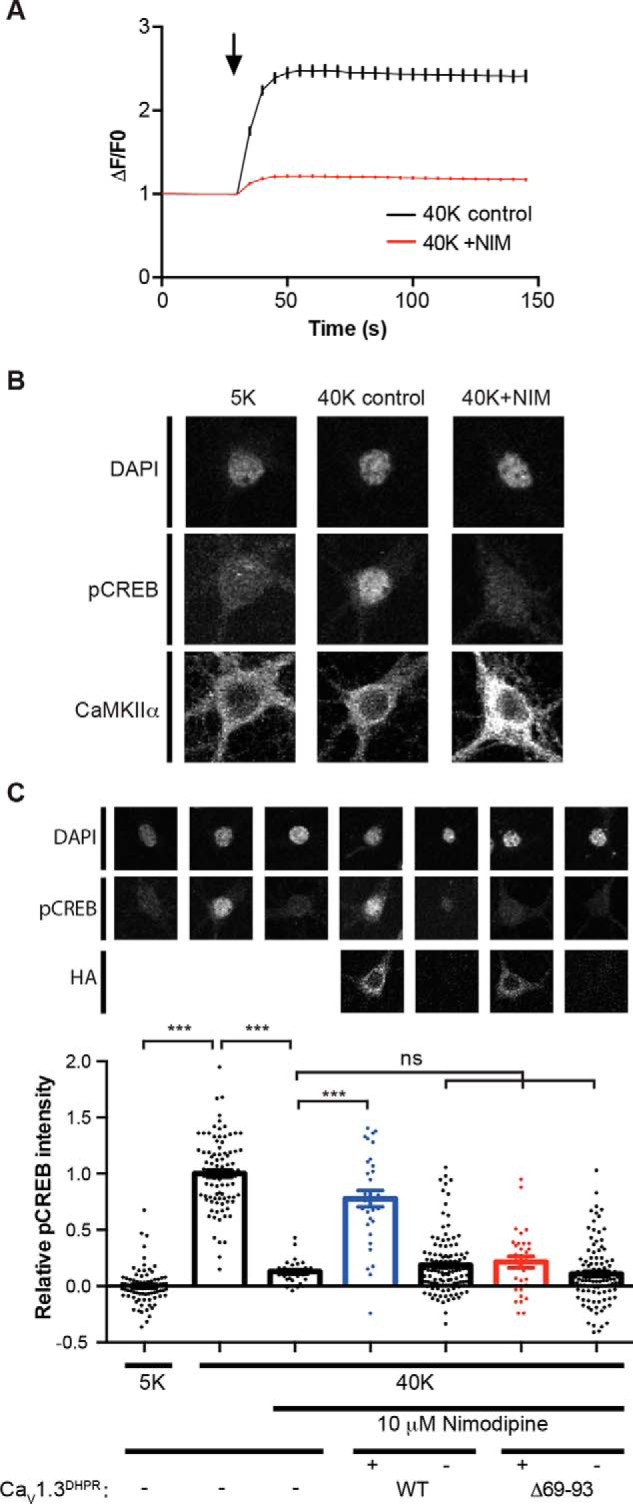
Role of the LTCC NTD in high K+-induced CREB Ser133 phosphorylation. A, Ca2+ imaging showing that nimodipine largely prevents the high K+-induced increase of somatic Ca2+. Neurons were incubated with Tyrode's solution containing 5 mm KCl (5K) for 30 s and switched to Tyrode's solution containing 40 mm KCl for 2 min in the absence (40K control, n = 193) or presence (40K + NIM, n = 215) of 10 μm nimodipine. The black arrow indicates the buffer switch, and data were plotted as mean ± S.E. A total of five dishes from two independent cultures were analyzed per group. B, depolarization of cultured hippocampal neurons induced LTCC-dependent Ser133 phosphorylation of CREB. In the columns from left to right, neurons were incubated with 5K Tyrode's solution and then switched to 5K, 40K control, or 40K + NIM Tyrode's solution, respectively, for 90 s. Neurons were then fixed and stained for DAPI, CREB Ser133 phosphorylation (pCREB), and CaMKIIα. Incubation with 40 mm KCl induced an increase of pCREB that was blocked by the LTCC antagonist nimodipine. C, deletion of the CaMKII-binding domain in the CaV1.3 NTD disrupts nuclear signaling. Expression of a nimodipine-resistant CaV1.3-T1033Y mutant rescues the nimodipine blockade of pCREB induction by 40 mm KCl. However, deletion of the CaMKII-binding domain (Δ69–93) prevents the rescue of pCREB signaling by CaV1.3-T1033Y. Each data point represents analysis of a single cell collected from 3–4 independent neuronal cultures/transfections. Pooled data were analyzed by one-way ANOVA followed by Tukey's multiple-comparison test. ***, p < 0.001; ns, not significant (p > 0.05). All confocal images show a 40 × 40-μm area. Error bars, S.E.
