Figure 8.
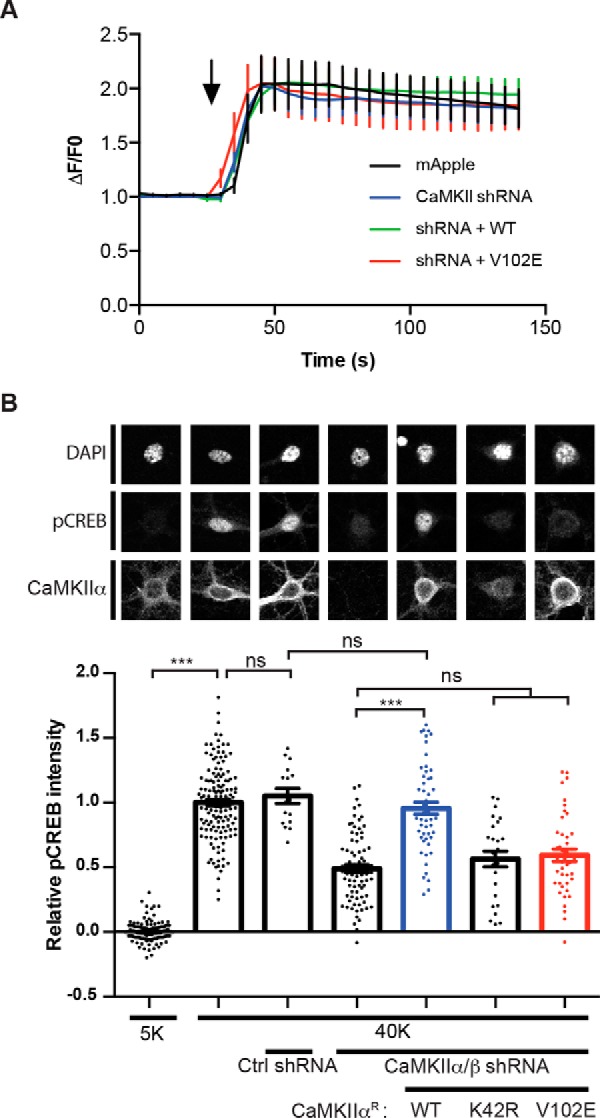
CaMKII-binding to the CaV1.3 NTD is required for high K+-induced CREB Ser133 phosphorylation. A, CaMKIIα/β knockdown or re-expression/rescue has no effect on high K+-induced increases of somatic Ca2+. Cultured hippocampal neurons were transfected with mApple only (n = 17), mApple with CaMKIIα/β shRNA (n = 23), mApple/CaMKIIα/β shRNA with shRNA-resistant CaMKIIαR-WT (n = 26), or CaMKIIαR-V102E cDNA (n = 17), respectively. Transfected neurons were then monitored for Ca2+ influx in response to 40 mm K+-induced depolarization (black arrow; see Fig. 7A). B, the CaMKIIα-V102E mutant does not support nuclear signaling. Expression of CaMKIIα/CaMKIIβ shRNAs significantly reduces nuclear CREB phosphorylation following 40 mm KCl treatment. This reduction in CREB phosphorylation is rescued by co-expression of shRNA-resistant CaMKIIαR-WT, but not CaMKIIαR-K42R (kinase-dead) or CaMKIIαR-V102E (deficient in CaV1.3 NTD binding). Each data point represents analysis of a single cell collected from 3–4 independent neuronal cultures/transfections. Pooled data were analyzed by one-way ANOVA followed by Tukey's multiple-comparison test. ***, p < 0.001; ns, not significant (p > 0.05). All confocal images show a 40 × 40-μm area. Error bars, S.E.
