Figure 4.
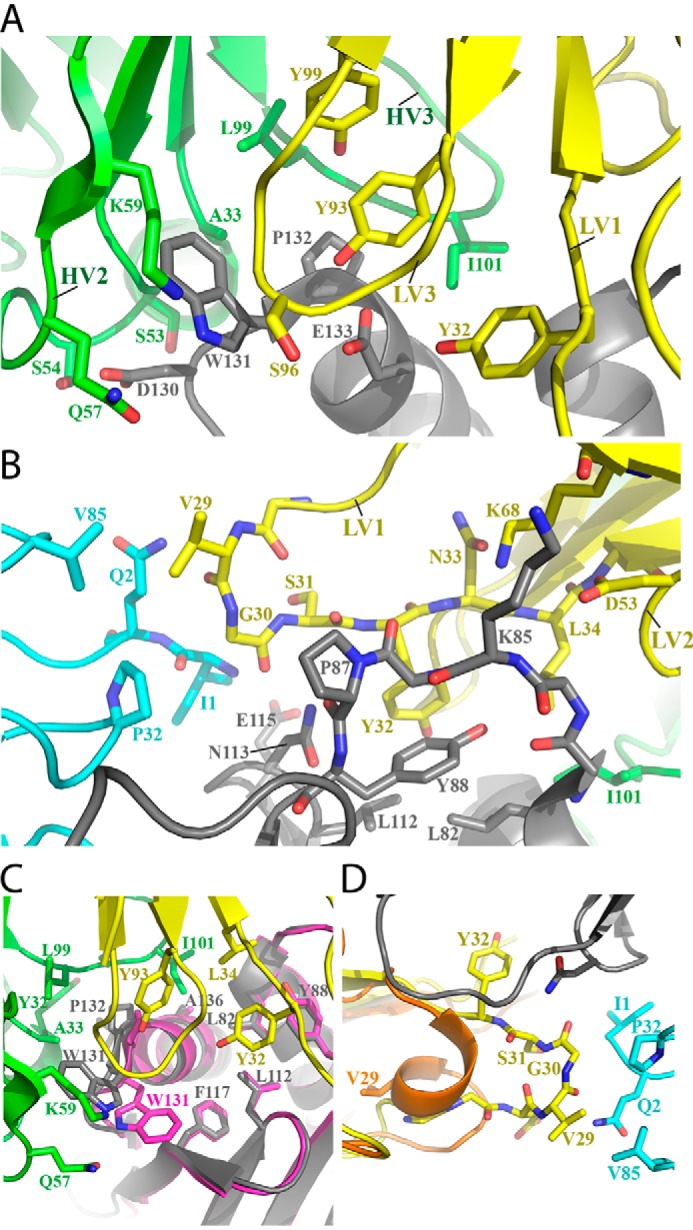
A detailed look at the DX-2507–FcRn complex paratope–epitope interface. A, the central interaction between DX-2507 HV2, HV3, LV1, and LV3 and the FcRn stretch of residues 130DWPE133 drives complex formation. The color scheme is as in Fig. 3. B, DX-2507 interacts with a second major linear stretch of FcRn residues Ala81–Tyr88. C, a view of the extensive hydrophobic interactions that define the center of the complex interface. In addition, it is notable that Trp131 is flipped toward the DX-2507 paratope relative to all other reported human FcRn structures, including the Fc-complexed FcRn structure aligned here (magenta; PDB code 4N0U). D, DX-2507 LV1 unfurls from an α-helical conformation in the apo-Fab structure (orange) to an extended loop, as the complexed Fab (yellow) now contacts residues in the β2M subunit of FcRn (cyan).
