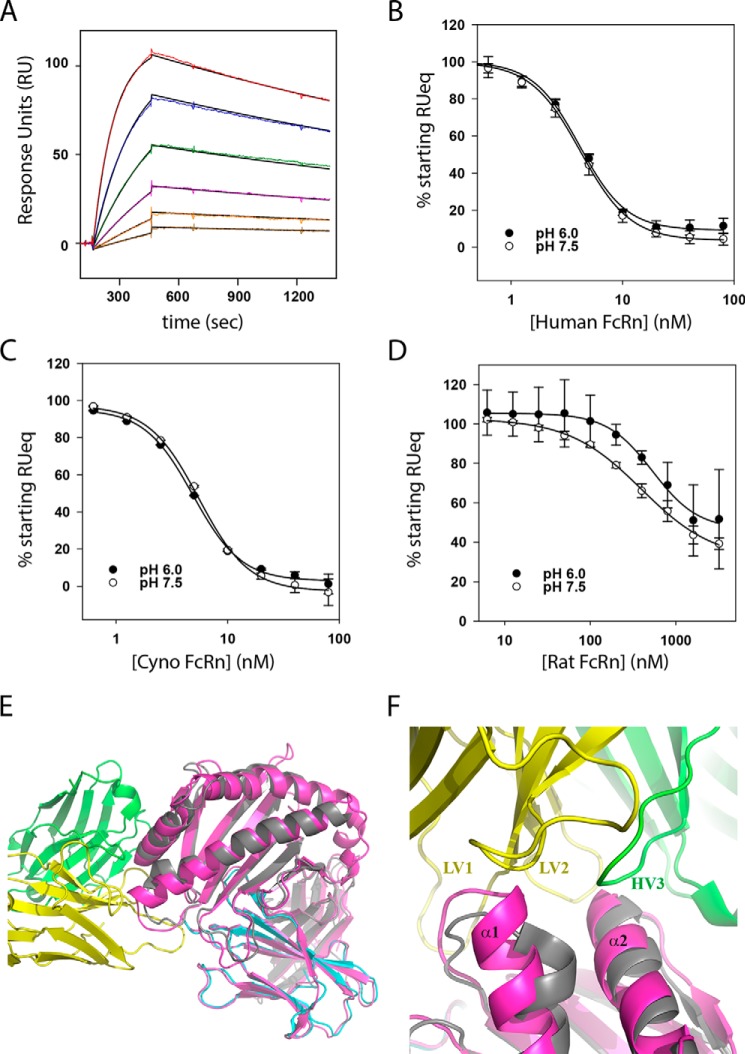
Figure 5.
DX-2507 potently binds cyno FcRn but not rat FcRn. A, an example SPR sensorgram of soluble cyno FcRn interacting with surface-immobilized DX-2507 Fab. Color lines show the experimental RU value for the association and dissociation phases, with the solid black line representing the best fit to a 1:1 Langmuir model. B, a competition SPR experiment probes the ability of added FcRn to compete away binding of DX-2507 Fab analyte to surface-immobilized human FcRn. Error bars, S.D. of replicates (n ≥ 2); solid lines, fit to an IC50 equation = 4.1 ± 0.3 nm (pH 6.0) and 4.2 ± 0.2 nm (pH 7.5). C, competition SPR as in B but with increasing concentrations of cyno FcRn as the competitor. Fitted IC50 = 4.9 ± 0.2 nm (pH 6.0) and 5.5 ± 0.2 nm (pH 7.5). D, rat FcRn competition SPR demonstrates a significant decrease in affinity for this species, with resulting IC50 fits = 535 ± 60 nm (pH 6.0) and 397 ± 33 nm (pH 7.5). E, alignment of the DX-2507–shFcRn complex structure (colored as in Fig. 3) with rat FcRn (colored magenta; PDB code 3FRU) shows generally good agreement between the structures (RMSD = 1.07 Å). F, close-up of the alignment of rat and human FcRn at the DX-2507 interface reveals a small but significant extension of α-helix 1 in the rat FcRn that would protrude into DX-2507 LV1 and LV2, probably explaining why this interaction has reduced affinity relative to human FcRn.