FIGURE 2.
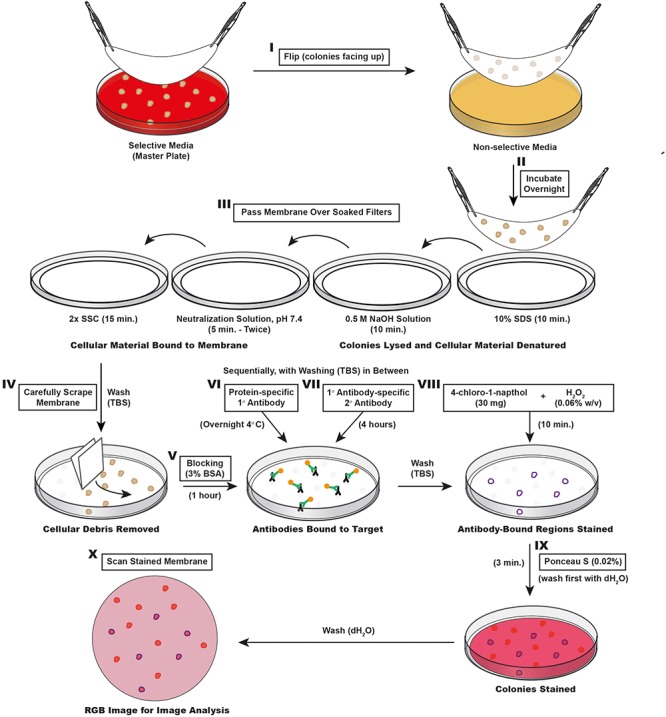
Schematic outline of the ColoBlot procedure. (I) Colonies grown on the master plate are transferred on a nitrocellulose membrane. (II) The membrane is then placed colony side up on a non-selective agar plate and incubated overnight. (III) The membrane is passaged over different buffer soaked Whatman papers to lyse colonies and bind cellular material to the membrane. (IV) The membrane is washed and excess cellular debris is removed by lightly scraping the membrane with Whatman paper. (V) Another round of washing is followed by blocking in TBS buffer containing 3% BSA. (VI) Protein-specific primary antibodies are added to the membrane in blocking solution. (VII) After three rounds of washing, HRP-conjugated secondary antibodies are added in blocking solution. Another three rounds of washing removes antibody in excess. (VIII) The staining was performed by exposing the membrane to 4-chloro-1-naphthol in presence of H2O2. This reaction is terminated after 10 min by washing with dH2O. (IX) A non-specific counter stain is performed by incubating the membrane in the presence of a Ponceau S solution. Destaining (to increase the signal-to-background ratio) is achieved with sequential washing with dH2O. (X) After drying, the membrane is ready to be scanned as an RGB TIFF image for CIIA.
