Table 1. Metal-free catalytic dehydrogenation of HCOOH/NEt3 (5 : 2) a .
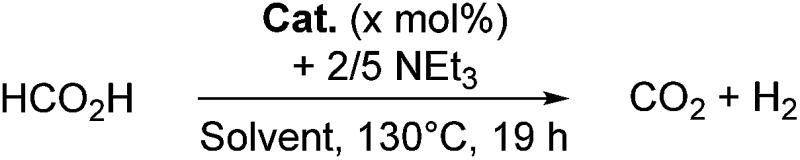
| ||||
| Entry | Catalyst (amount, mol%) | Solvent | Conv. b [%] | TON (time, h) |
| 1 | [2+ , I–] (5.0) | TDF | 26 | 5.2 (19) |
| 2 | BBN–I (5.0) | TDF | 48 | 9.6 (19) |
| 3 | BBN–I (5.0) | C6D6 | <5 | — |
| 4 | BBN–I (5.0) | Tol-d 8 | <5 | — |
| 5 | BBN–I (5.0) | CD3OD | <5 | — |
| 6 | BBN–I (5.0) | CD3CN | 84 | 16.8 (19) |
| 7 | BBN–H (5.0) | CD3CN | 52 | 10.4 (19) |
| 8 | [TBDH+, 5– ] (5.0) | CD3CN | 67 c | 13.4 (19) |
| 9 | Cy2B–I (10.0) | CD3CN | >99 d | 10 (4.5) |
| 10 | Cy2B–I (5.0) | CD3CN | >99 | 20 (19) |
| 11 | Cy2B–I (1.0) | CD3CN | 79 | 79 (19), 88 (23), 100 (40) |
| 12 | [Et3NH+, 6– ] (5.0) | CD3CN | >99 c | 20 (8) |
| 13 | [Et3NH+, 6– ] (1.0) | CD3CN | 78 c | 78 (19), 94 (23), 100 (26) |
aReaction conditions: 0.2 mmol FA, 0.08 mmol TEA, 0.2 mL deuterated solvent.
bConversions determined by 1H NMR using mesitylene (10 μL) as an internal standard; mean values over at least 2 runs.
cCatalyst was recovered unchanged.
dFull conversion reached within 4.5 h.
