Table 2. Catalytic hydroamination of alkynes with primary amines a .
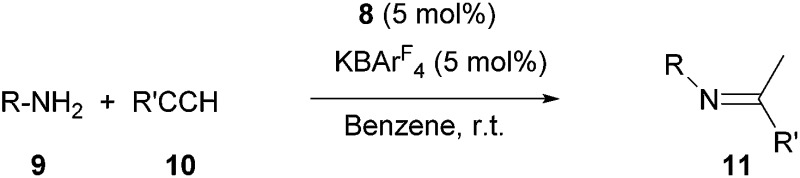
| ||||
| Entry | 9 | 10 | Time (h) | 11, Yield b (%) |
| 1 | 9a (R = Ph) | 10a (R′ = Ph) | 24 | 11aa, 98 |
| 2 | 9a | 10b (R′ = 4-MeOC6H4) | 24 | 11ab, 93 |
| 3 | 9a | 10c (R′ = 4-BrC6H4) | 72 | 11ac, 86 |
| 4 | 9b (R = 4-MeOC6H4) | 10a | 36 | 11ba, 90 |
| 5 | 9a | 10b | 36 | 11bb, 94 |
| 6 | 9a | 10c | 72 | 11bc, 54 |
| 7 | 9c (R = 3,5-Me2C6H3) | 10a | 36 | 11ca, 91 |
| 8 | 9d (R = 2,4,6-Me3C6H2) | 10b | 72 | 11db, 73 |
| 9 | 9e (R = 2,6-iPr2C6H3) | 10a | 72 | 11ea, 46 |
| 10 | 9e | 10b | 72 | 11eb, 44 |
| 11 | 9e | 10c | 72 | 11ec, 30 |
aReaction conditions: 9 (2 mmol), 10 (2 mmol) benzene (3 mL).
bIsolated yields.
