Abstract
Background
To describe trajectories of health‐related quality of life (QoL), life satisfaction, and psychological adjustment for men with prostate cancer over the medium to long term and identify predictors of poorer outcomes using growth mixture models.
Methods
One‐thousand sixty‐four (82.4% response) men diagnosed with prostate cancer were recruited close to diagnosis and assessed over a 72‐month (6‐year) period with self‐report assessment of health‐related QoL, life satisfaction, cancer‐related distress, and prostate specific antigen anxiety. Urinary, bowel, and sexual function were also assessed using validated questionnaires.
Results
Poorer physical QOL was predicted by older age, lower education, lower income, comorbidities, and receiving hormone therapy. Lower life satisfaction was related to younger age, lower income, not being partnered, and comorbidities. Poorer psychological trajectories were predicted by younger age, lower income, comorbidities, and receiving radical prostatectomy or brachytherapy. Better urinary, bowel, and sexual function were related to better global outcomes over time. Anxiety about prostate specific antigen testing was rare.
Conclusions
Distinct trajectories exist for medium‐ to long‐term QoL, life satisfaction, and psychological adjustment after prostate cancer; with age and socioeconomic deprivation playing a differential role in men's survivorship profile and the impact of functional status on outcomes increasing over time. These results reinforce the need for an appraisal of men's life course in addition to treatment side effects when planning survivorship care after cancer.
Keywords: cancer, longitudinal, oncology, prostate, psychological distress, quality of life
1. INTRODUCTION
Prostate cancer is the most common cancer among men in developed countries, with an estimated 740 000 men diagnosed in these countries in 2012 alone (75% of global incidence).1 Five‐year relative survival for this cancer now approaches 100% in some countries for those diagnosed at a localised stage,2 although there were still an estimated 140 000 deaths due to prostate cancer in developed countries in 2012.1 There is often a long time frame between prostate cancer diagnosis and death, with men continuing to have excess mortality up to 15 years after diagnosis,3 and dying from their prostate cancer more than 20 years after diagnosis.4 This means prostate cancer survivorship for many men is a long‐lived experience, with quality of life (QoL) and psychological well‐being crucial considerations in care after diagnosis. To date, there is limited data on long‐term survivorship (>5 years post diagnosis) in general, and this is more so with regards to the survivorship experience of men with prostate cancer.5
To date, the domain‐specific QoL effects of treatment for prostate cancer have been well described. These include urinary, bowel, and sexual dysfunction, as well as effects specific to hormone ablation therapy with these effects differing by treatment approach. For example, 5 years after diagnosis men treated with radical prostatectomy (RP) experience worse urinary incontinence, but similar sexual outcomes, compared to men treated with radiation therapy.6 By contrast, men treated with radiation therapy experience more long‐term adverse effects on bowel function with androgen deprivation having the greatest adverse effect on physical QoL.7 Studies relating to overall health‐related QoL suggest that men's mental and physical wellbeing are stable in the medium to long term. However, the predominant focus on domain‐specific effects leaves gaps in our understanding of the impact of prostate cancer on men's global QoL and may obscure patient subgroups who do not fare well over time.
Even less is known about the course of long‐term psychological adjustment after prostate cancer, with distress estimates close to diagnosis varying from 10% to 23%.8 Depression and anxiety seem to be highest at treatment commencement for men with localised and locally advanced prostate cancer, reducing to levels below or similar to that of the general population after 12 months.9 To date, there is scant research examining long‐term (>5 years) psychological adjustment after prostate cancer, with limitations including the use of non‐validated asesssments.10 For those studies that did apply well validated measures, 1 group reported that 10% of men with localised prostate cancer experience clinically significant depression up to 8 years after treatment.11 By contrast, Korfage et al (2006) reported that one in 4 men (28%) with prostate cancer were highly anxious before treatment; although by 6 months, this reduced with most men experiencing low distress at 5 years.12 These studies however were not able to assess for heterogeneity in trajectories of adjustment and so were limited in their ability to longitudinally identify risk factors for distress and patterns of change.
In this regard, growth mixture models (GMMs) have been widely used to assess for heterogeneity of trajectories of scores within a population.13 Growth mixture models allow different statistical distributions for analysing various types of score variables, the identification of differential features among trajectory classes, and the inclusion of time‐varying covariates for modelling the association between the score variable and predictors at each measurement time point.14, 15 By applying this approach in our previous research with colorectal cancer patients, we identified unique patterns of risk that were associated with distinct psychological adjustment and QoL trajectories.16, 17 To date, to our knowledge, this approach has not been applied to examine men's adjustment to prostate cancer.
Thus, our aim in the current study was to examine the trajectories of health‐related QoL, life satisfaction, and psychological adjustment in men previously diagnosed with prostate cancer who were followed over a 6‐year period. We examined socio‐demographic predispositional and clinical factors as well as time varying functional outcomes as predictors to identify characteristics of men “at risk” for poorer outcomes across treatment types.
2. METHOD
2.1. Participants
These data are from a longitudinal study of men newly diagnosed with prostate cancer in Queensland.18, 19, 20 Ethical approval was obtained from the Queensland University of Technology Human Research Ethics Committee and ethics committees of 10 public hospitals in Queensland. Men newly diagnosed with prostate cancer from the geographic catchment areas of South East and North Queensland, Australia, were recruited between March 2005 and September 2007. Eligibility criteria included ability to read, write, and speak English; no history of dementia, head injury or psychiatric illness; and regular access to a telephone. Urologists referred men to the study if they were newly diagnosed with prostate cancer irrespective of stage of disease. Of the 1291 men referred to the study, 1064 (82.4%) were eligible and consented to participate. Participants completed assessments at baseline, and 2, 6, 12, 24, 36, 48, 60, and 72 months after the start of treatment. Baseline assessments were completed for 1034 (97%) men. In all, 575 (54% from eligible and consented) men completed assessments at all 9 time points, with 357 men withdrawing from the study between baseline and 72 month assessment. Self‐report measures were administered by mail at each time point. Of the total sample, 41 men had died at 6 years. Other reasons for attrition included being too unwell, not contactable, and not wanting to think about prostate cancer anymore.
2.2. Predictor variables
Socio‐demographic variables including age, marital status, education and income, and clinical variables such as chronic health conditions, treatment type, time since diagnosis, prostate specific antigen (PSA), Gleason score, and stage were collected at baseline.
2.3. Outcome variables
2.3.1. Quality of life
The Short Form 36 (SF‐36) assessed health‐related QoL.21 Two global measures of psychological and physical functioning are derived (mental health domain and physical health domain) whereby higher scores indicate better health‐related QoL.
Disease‐specific QoL was measured using the expanded prostate cancer index composite (EPIC)22 assessing urinary, bowel, and sexual function domains. All EPIC scores were standardised to a 0 to 100 scale. Higher scores indicate better QoL. Internal reliability among the 3 EPIC function summary scores ranged from α = 0.76 to 0.95 (urinary, α = 0.83 to 0.86; bowel, α = 0.83 to 0.90; and sexual, α = 0.90 to 0.95).
2.3.2. Satisfaction with life
The satisfaction with life scale (SWL)23 assessed participants' subjective cognitive well‐being (α = 0.88 to 0.91). Higher scores indicate a higher satisfaction with life.
2.3.3. Cancer‐related distress
The revised impact of event scale (RIES)24 assessed cancer‐specific distress at baseline through to 60 months. The RIES contains 3 subscales: intrusion (α = 0.87 to 0.91), avoidance (α = 0.84 to 0.87), and hyperarousal (α = 0.83 to 0.87). Higher scores indicate higher cancer‐specific distress. The RIES was not administered after 5 years to reduce participant burden.
The PSA anxiety subscale of the memorial anxiety for prostate cancer (MAX‐PC) scale25, 26 assessed distress related to PSA testing (α = 0.60 to 0.77). Higher scores indicate higher distress.
2.4. Statistical analyses
Growth mixture models in Mplus Version 6.1215, 27 were used to identify trajectory classes and predictors of membership in these classes, separately for the QoL SF36 (physical health domain and mental health domain), the SWL score, and the cancer‐related distress RIES score. Nonlinear GMMs (consisting of intercept, slope, and quadratic growth parameters) were adopted to model individual growth trajectories from unobserved (latent) subpopulations, where individual variation in growth parameters (intercept and slope) was captured by random effects with different variance components.14, 28 The association between the EPIC (urinary, bowel, and sexual) functions and outcome variables at each of the time points was modelled by including the 3 function subscales in the GMMs as time‐varying covariates at each time point.13
The RIES scores were heavily right‐skewed, with around 10‐45% of 0 scores at each of the time points. Thus, the GMMs with Poisson and negative binomial distributions (including 0‐inflated models) were considered for the longitudinal analysis of the RIES scores over time.15 Model selection was determined using the Bayesian information criterion.29
With GMMs, the initialization of growth parameters was obtained by implementing a latent class growth analysis (assuming no within‐class variance) fitted to the data. The GMM analyses were implemented with 100 random sets of starting values and 10 final optimizations. The number of trajectory classes K was determined using the Lo‐Mendell‐Rubin likelihood ratio test statistic.30 Covariates were then entered into the K‐class (unconditional) GMM via multinomial logistic regression that compares the reference class (high‐SF36 domain or SWL scores, or low RIES score) with the other trajectory classes (lower SF36 domain or SWL scores, or higher RIES scores).
Missing data in outcome variables (SF36 domain, SWL, and RIES scores) were handled in Mplus using a robust full information maximum likelihood estimation procedure with the missing at random assumption that the missing scores are unrelated to the outcome variables.30 Estimates of covariance coverage for each pair of variables were checked for evaluating the impact of missing data in outcome variables on model convergence. Missing values in the EPIC time‐varying covariates were computed using the multiple imputation method with restriction to a 0 to 100 scale.31 In the first stage, the missing EPIC subscales were imputed based on the characteristics of the observed EPIC subscales, and this procedure was repeated 10 times to generate 10 imputed data sets. In the second stage, the GMMs were fitted separately to each of the imputed data sets and the results were pooled into a final set of estimates.31, 32 Individuals (N = 120) with more than 6 missing values in the EPIC time‐varying covariates were excluded for the analyses. This reduced the sample size from 1064 to 944 in the GMMs. Patients with missing values in other covariates were also excluded in the analyses.
Due to the fact that the proportion of 0 MAX‐PC scores was very high at each time point (79‐93%), it is not possible to obtain a robust estimation of GMMs (with 0‐inflated models) for the longitudinal analysis of MAX‐PC scores. Thus, descriptive statistical analyses were performed to illustrate the distribution of MAX‐PC scores over time.
3. RESULTS
Demographic and clinical characteristics of the study samples are provided in Table 1. The mean age of men at recruitment was 63.7 years; 82.7% were married with the remainder divorced, widowed, or never married; 53.3% had completed trade or university education, and the remainder had high school or less schooling; 43% had an annual income of $40 000AUS or less, 24.7% between $40 000 to $80 000AUS and 21.9% > $80 000AUS. The average self‐reported PSA level at baseline was 11.0; the average Queensland cancer registry Gleason score was 7; the average time since diagnosis at recruitment was 142 days; 92.1% of men had localized stage disease; 6.3% had locally advanced stage; only 1.6% had advanced stage; 46.3% had received a RP; 38.3% had external beam radiation therapy; and 16.7% had received brachytherapy (BT).
Table 1.
Demographic and clinical characteristics of study samples (N = 1064)
| Characteristics | Count (%) or Mean (SD) |
|---|---|
| Age at recruitment (years) | 63.7 (7.8) |
| Marital status | |
| Married/defacto | 880 (82.7%) |
| Divorced/separated | 110 (10.4%) |
| Widowed | 27 (2.5%) |
| Never married | 47 (4.4%) |
| Education level | |
| Primary school/not complete primary | 145 (13.6%) |
| Junior high school | 247 (23.3%) |
| Senior high school | 104 (9.8%) |
| Trade/diploma | 366 (34.5%) |
| University | 200 (18.8%) |
| Missing | 2 |
| Household income | |
| <$40 000 | 454 (43.0%) |
| $40 000‐$80 000 | 261 (24.7%) |
| >$80 000 | 231 (21.9%) |
| Not answer/don't know | 110 (10.4%) |
| Missing | 8 |
| PSA level at diagnosis | 11.0 (27.5) |
| Missing | 151 |
| QCR Gleason score | 7.0 (0.9) |
| Missing | 70 |
| Time since diagnosis at recruitment (days) | 142 (254) |
| Stage | |
| Localized | 978 (92.1%) |
| Locally advanced | 67 (6.3%) |
| Advanced | 17 (1.6%) |
| Missing | 2 |
| Comorbidity | |
| 0 conditions | 175 (16.6%) |
| 1‐2 conditions | 556 (52.8%) |
| ≥3 conditions | 323 (30.6%) |
| Missing | 10 |
| Therapy performed | |
| Radical prostatectomy | 493 (46.3%) |
| External beam radiation | 407 (38.3%) |
| Brachytherapy | 178 (16.7%) |
| Hormone therapy | 385 (36.2%) |
| Watchful waiting | 59 (5.5%) |
Abbreviations: PSA, prostate specific antigen; QCR, Queensland cancer registry.
3.1. Quality of life: physical health
Three distinct classes of trajectory patterns were identified for the QoL SF36 (physical health domain) using GMM (Figure 1A). The constant high QoL class (Class 3) indicates a group of patients (50.3%) who had constantly high physical health throughout the 6‐year follow‐up period. Class 2 represents a group of patients (30.2%) whose physical health started at a medium level and then at 3 years post‐diagnosis began to decrease. Class 1 (19.5% of patients) indicates a physical health trajectory pattern, which decreased steadily from a medium level and then increased at 4 years post‐diagnosis.
Figure 1.
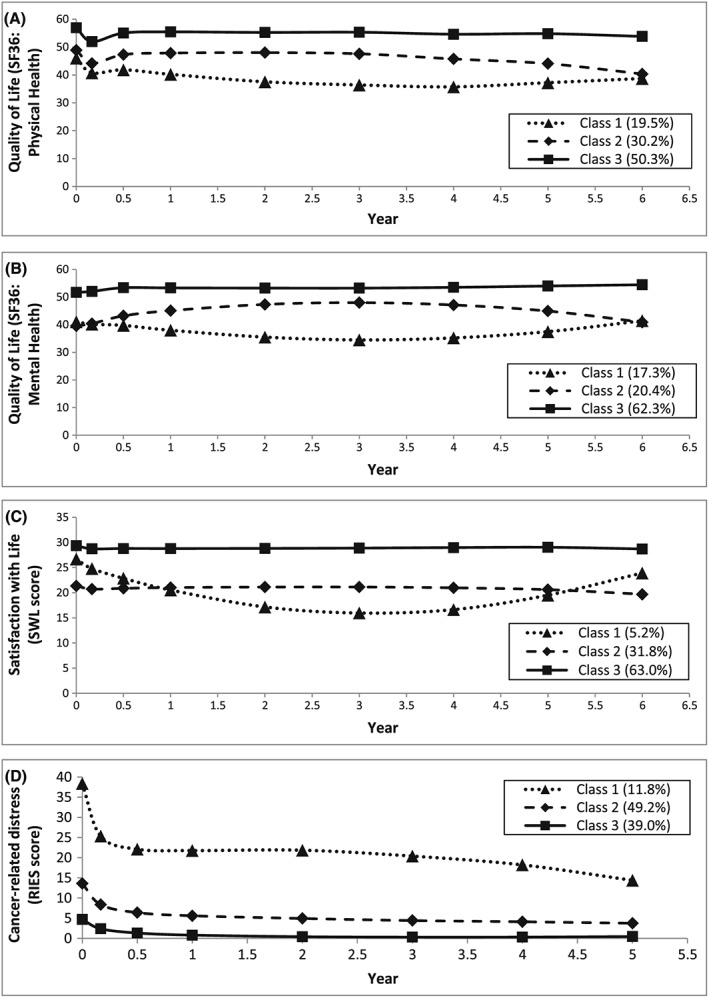
Trajectory patterns identified using growth mixture models: A, quality of life SF36, physical health domain (N = 928); B, quality of life SF36, mental health domain (N = 928); C, satisfaction with life (SWL) score (N = 928); D, cancer‐related distress revised impact of event scale (RIES) score (N = 934)
Three predictors that significantly (P < .05) differentiated the physical health trajectory classes with the constant high QoL (Class 3) as the comparison class were identified within the GMMs (Table 2A). In comparison with the constant high QoL class, Class 2 (medium decrease) was characterized by patients with high levels of comorbidity (adjusted odds ratio [OR] = 2.26, 95% confidence interval [CI] = 1.09‐4.68 for 1‐2 conditions; adjusted OR = 6.81, 95% CI = 2.80‐16.5 for ≥3 conditions) and patients who did not receive RP (adjusted OR = 0.24, 95% CI = 0.10‐0.57) or BT (adjusted OR = 0.24, 95% CI = 0.11‐0.55). Class 2 was also characterized by patients who received hormone therapy (adjusted OR = 2.39, 95% CI = 1.08‐5.31). Compared to the constant high QoL class, Class 1 was differentiated by patients with lower household income (adjusted OR = 12.76, 95% CI = 2.48‐65.7 for income <$40 000; adjusted OR = 9.15, 95% CI = 1.70‐49.3 for income between $40 000 and $80 000), patients with ≥3 comorbid conditions (adjusted OR = 9.51, 95% CI = 3.84‐23.5), and patients who did not receive RP (adjusted OR = 0.13, 95% CI = 0.05‐0.34) or BT (adjusted OR = 0.25, 95% CI = 0.10‐0.59). Moreover, Class 1 was characterized by patients who received hormone therapy (adjusted OR = 2.95, 95% CI = 1.29‐6.71).
Table 2.
Predictors of trajectory class membership for various outcome measures
| Predictor | Adjusted OR (95% CI) relative to constant high class (Class 3) | P valuea | |
|---|---|---|---|
| Class 2 | Class 1 | ||
| (A) Quality of life SF36 (physical health domain, N = 928) | |||
| Household income | <.001 | ||
| <$40 000 | 1.83 (0.96, 3.47) | 12.76* (2.48, 65.7) | |
| $40 000‐$80 000 | 1.33 (0.59, 3.00) | 9.15* (1.70, 49.3) | |
| >$80 000 | Reference | Reference | |
| Not answer/do not know | 1.71 (0.70, 4.20) | 5.30 (0.83, 33.8) | |
| Comorbidity | <.001 | ||
| 0 condition | Reference | Reference | |
| 1‐2 conditions | 2.26* (1.09, 4.68) | 1.99 (0.88, 4.52) | |
| ≥3 conditions | 6.81* (2.80, 16.5) | 9.51* (3.84, 23.5) | |
| Therapy performed vs nil | <.001 | ||
| Radical prostatectomy | 0.24* (0.10, 0.57) | 0.13* (0.05, 0.34) | |
| External beam radiation | 1.02 (0.51, 2.04) | 0.43 (0.18, 1.00) | |
| Brachytherapy | 0.24* (0.11, 0.55) | 0.25* (0.10, 0.59) | |
| Hormone therapy | 2.39* (1.08, 5.31) | 2.95* (1.29, 6.71) | |
| Watchful waiting | 1.12 (0.41, 3.08) | 0.49 (0.16, 1.56) | |
| (B) Quality of life SF36 (mental health domain, N = 928) | |||
| Household income | .008 | ||
| <$40 000 | 1.44 (0.74, 2.82) | 2.55* (1.30, 5.00) | |
| $40 000‐$80 000 | 1.40 (0.72, 2.71) | 1.33 (0.62, 2.85) | |
| >$80 000 | Reference | Reference | |
| Not answer/don't know | 0.84 (0.32, 2.24) | 1.33 (0.49, 3.61) | |
| Comorbidity | <.001 | ||
| 0 conditions | Reference | Reference | |
| 1‐2 conditions | 2.69* (1.18, 6.12) | 1.71 (0.82, 3.58) | |
| ≥3 conditions | 3.84* (1.51, 9.75) | 3.26* (1.50, 7.07) | |
| (C) Satisfaction with life (SWL, N = 928) | |||
| Age (younger) | 1.10* (1.06, 1.13) | 1.00 (0.93, 1.08) | <.001 |
| Marital status | <.001 | ||
| Married/defacto | Reference | Reference | |
| Never married/widowed/divorced/separated | 2.68* (1.61, 4.48) | 1.07 (0.34, 3.33) | |
| Household income | .022 | ||
| <$40 000 | 2.24* (1.24, 4.05) | 1.85 (0.60, 5.74) | |
| $40 000‐$80 000 | 1.40 (0.81, 2.44) | 0.80 (0.24, 2.75) | |
| >$80 000 | Reference | Reference | |
| Not answer/don't know | 0.91 (0.41, 2.00) | 0.57 (0.10, 3.31) | |
| Comorbidity | <.001 | ||
| 0 conditions | Reference | Reference | |
| 1‐2 conditions | 1.74 (0.99, 3.04) | 0.83 (0.35, 1.98) | |
| ≥3 conditions | 3.17* (1.73, 5.80) | 0.29 (0.07, 1.28) | |
| (D) Cancer‐related distress (RIES, N = 934) | |||
| Age (younger) | 1.03* (1.00, 1.06) | 1.08* (1.04, 1.13) | <.001 |
| Comorbidity | <.001 | ||
| 0 conditions | Reference | Reference | |
| 1‐2 conditions | 1.02 (0.65, 1.58) | 3.56* (1.31, 9.65) | |
| ≥3 conditions | 1.09 (0.64, 1.85) | 5.57* (1.92, 16.1) | |
| Therapy performed vs nil | .003 | ||
| Radical prostatectomy | 2.28* (1.11, 4.69) | 0.56 (0.17, 1.81) | |
| External beam radiation | 2.30* (1.35, 3.91) | 0.54 (0.21, 1.36) | |
| Brachytherapy | 0.98 (0.56, 1.71) | 0.39 (0.13, 1.17) | |
| Hormone therapy | 1.15 (0.64, 2.09) | 1.38 (0.65, 2.94) | |
| Watchful waiting | 2.14 (0.90, 5.12) | 0.77 (0.15, 4.01) | |
Abbreviations: CI, confidence interval; OR, odds ratio; RIES, revised impact of event scale; SWL, satisfaction with life scale.
significant at 0.05 level on the adjusted log odds of being in the class versus Class 3
likelihood ratio test (full model versus model without the predictor under consideration)
3.2. Quality of life: mental health
Three distinct classes of trajectory patterns were identified for the QoL SF36 (mental health domain) using GMM (Figure 1B). The Constant High QoL class (Class 3) indicates a group of patients (62.3%) who had constantly high mental health throughout the 6 year follow‐up period. Class 2 represents a group of patients (20.4%) whose mental health increased initially and then at 3‐years post‐diagnosis began to decrease. Class 1 (17.3% of patients) indicates a mental health trajectory pattern which decreased initially and then increased at 3‐years post‐diagnosis.
Two predictors that significantly (P < .05) differentiated the mental health trajectory classes with the constant high QoL as the comparison class were identified within the GMMs (Table 2B). Compared to the constant high QoL class, Class 2 was characterized by patients with high levels of comorbidity (adjusted OR = 2.69, 95% CI = 1.18‐6.12 for 1‐2 conditions; adjusted OR = 3.84, 95% CI = 1.51‐9.75 for ≥3 conditions). Compared to the constant high QoL class, Class 1 was differentiated by patients with household income <$40 000 (adjusted OR = 2.55, 95% CI = 1.30‐5.00) and patients with ≥3 comorbid conditions (adjusted OR = 3.26, 95% CI = 1.50‐7.07).
3.3. Satisfaction with life
Three distinct classes of trajectory patterns were identified for SWL using GMM (Figure 1C). The constant high SWL class (Class 3) indicates a group of patients (63.0%) who had constantly high SWL throughout the 6‐year follow‐up period. Class 2 represents a group of patients (31.8%) whose SWL was maintained at a low level constantly. Class 1 (5.2% of patients) indicates a SWL trajectory pattern, which decreased from a medium level and then increased at 3‐years post‐diagnosis.
Four predictors that significantly (P < .05) differentiated the SWL trajectory classes with the constant high satisfaction as the comparison class were identified within the GMMs (Table 2C). In comparison with the constant high satisfaction class, Class 2 (low constant) was characterized by patients who were never married/widowed/divorced/separated (adjusted OR = 2.68, 95% CI = 1.61‐4.48), younger age (adjusted OR = 1.10 per 1‐year decrease in age, 95% CI = 1.06‐1.13), and patients with household income <$40 000 (adjusted OR = 2.24, 95% CI = 1.24‐4.05) and ≥3 comorbid conditions (adjusted OR = 3.17, 95% CI = 1.73‐5.80).
3.4. Cancer‐related distress
Three distinct classes of trajectory patterns were identified for cancer‐related distress (RIES) using GMM with a negative binomial model (Figure 1D). Cancer‐related distress dropped significantly in the first half year of the follow‐up period for all 3 classes. The low cancer‐related distress class (Class 3) indicates a group of patients (39.0%) who had low cancer‐related distress throughout the 5 year follow‐up period. Class 2 represents a group of patients (49.2%) whose cancer‐related distress maintained at a medium level. Class 1 (11.8% of patients) indicates a cancer‐related distress trajectory pattern, which was maintained at a higher level.
Three predictors that significantly (P < .05) differentiated the cancer‐related distress trajectory classes with the low RIES score as the comparison class were identified within the GMMs (Table 2D). In comparison with the low RIES class, Class 2 (medium RIES) was characterized by patients who were younger in age (adjusted OR = 1.03 per 1‐year decrease in age, 95% CI = 1.00‐1.06) and who received RP (adjusted OR = 2.28, 95% CI = 1.11‐4.69) or BT (adjusted OR = 2.30, 95% CI = 1.35‐3.91). Compared to the low RIES class, Class 1 (high RIES) was differentiated by patients who were younger in age (adjusted OR = 1.08 per 1‐year decrease in age, 95% CI = 1.04‐1.13) and had high levels of comorbidity (adjusted OR = 3.56, 95% CI = 1.31‐9.65 for 1‐2 conditions; adjusted OR = 5.57, 95% CI = 1.92‐16.1 for ≥3 conditions).
3.5. PSA anxiety
The proportion of 0 MAX‐PC scores or PSA anxiety was high at each time point. In brief, 79.1% of patients had 0 PSA anxiety at baseline, and this increased gradually during the follow‐up period. At 6‐years post‐diagnosis, the proportion of men with no PSA anxiety was 92.6%. For those patients with PSA Anxiety (nonzero MAX‐PC scores), the mean score was 2.33 at baseline, which declined slightly during the follow‐up period (however, an increase of mean scores was observed at 1‐ and 4‐years post‐diagnosis). At 6‐years post‐diagnosis, the mean PSA Anxiety score was 2.08. The improvement in PSA anxiety was also reflected by the lowered maximum PSA anxiety among the patients. From 5‐years post‐diagnosis, there were no patients with PSA anxiety scores greater than 6.
3.6. Time‐varying effects of the EPIC subscales
The effects of the EPIC urinary, bowel, and sexual function on the 4 outcome variables at each of the time points are displayed in Figure 2. Generally, higher EPIC function significantly increased the patient's QoL, SWL, and reduced cancer‐related distress. The effects of bowel function on physical and mental health and cancer‐related distress were larger compared to urinary and sexual function. For cancer‐related distress, the effects of the EPIC functions were larger approximately 24‐36 months post‐diagnosis, relative to the effects just after diagnosis (Figure 2D). This indicates that the effects of function on cancer‐related distress increases over time.
Figure 2.
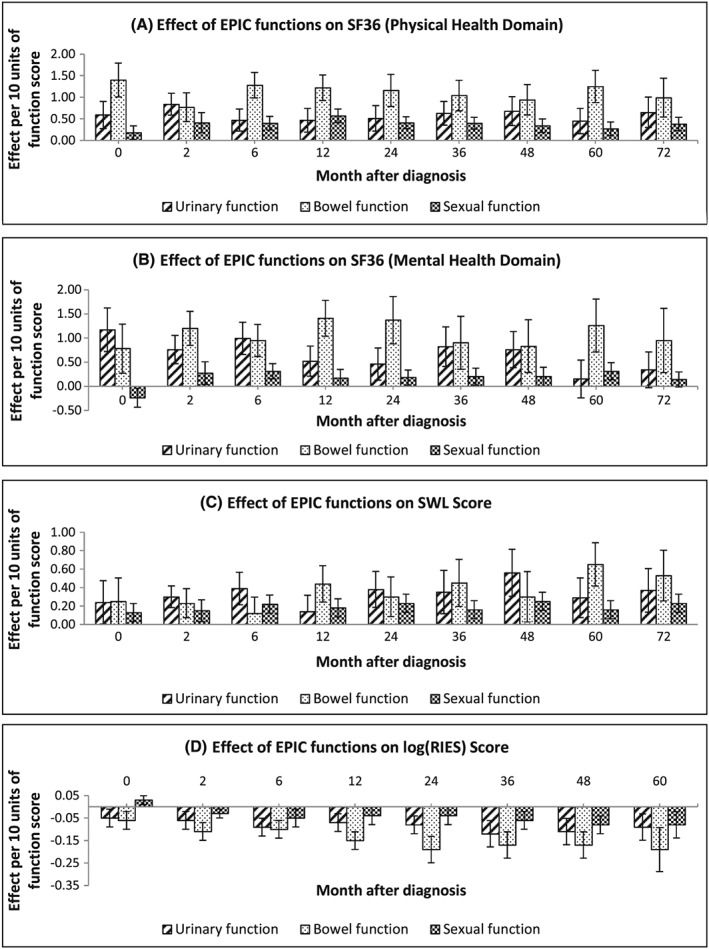
Effects from the expanded prostate cancer index composite (EPIC) urinary, bowel, and sexual functions on A, SF36 physical health, B, SF36 mental health, C, satisfaction with life (SWL), and D, revised impact of event scale (RIES): a positive value of effects means that a higher EPIC function increases the score of the outcome variable, while a negative value indicates that a higher EPIC function decreases the score of the outcome variable
4. DISCUSSION
The present results suggest particular characteristics for men with a diagnosis of prostate cancer that predict, over the medium and long term, poorer QoL and psychological outcomes. First, if men are younger at the time of diagnosis, they can be expected to be at greater risk for experiencing higher cancer‐related distress and poorer life satisfaction. There are a number of reasons why younger men may be more likely to experience these outcomes that reflect the greater disruption of life goals and circumstances when cancer occurs at a younger age.33 Younger men may still be building or consolidating careers, be more sexually active, and have greater financial responsibilities than older men. From this, the burden of cancer, and the uncertainty and psychological demands that come with living with cancer, may be heightened. Similar to previous research,34 older men were more at risk for poorer physical QoL that may link to the comorbidities associated with age that increase the physical challenges of treatment. Indeed, comorbidities themselves were associated with poorer physical and mental well‐being highlighting the burden of chronic illness more broadly in this patient population. For QoL, life satisfaction and psychological distress, the man's income and level of education were variously associated with poorer outcomes, reflecting the important role of economic advantage as a coping resource. This finding is also consistent with previous research showing men with low health literacy are more vulnerable to poorer mental health after prostate cancer.35 Finally, men who were un‐partnered were also at risk of greater psychological distress, with this a likely indicator of low social support. From this, we propose that an appraisal of a man's life course and situation is an essential first step for an effective and well‐targeted survivorship plan.33
We observed, over time, an increasing negative effect of poor sexual, urinary, and bowel function on cancer‐specific distress, peaking at 2‐ to 3‐years post diagnosis. Impacts of these areas of function for many men are long term, and these data suggest that rather than men learning to live with it, functional deficits over time may amplify avoidant and intrusive thinking about cancer, as well as hyperarousal or physical symptoms of distress. This is particularly striking given the relative absence in this large cohort of PSA anxiety, although this finding may reflect that rather than being pervasive, PSA anxiety is centred more around specific time points (eg, clinical checkups). Sexual, urinary, and bowel function therefore may be of more concern to men over the course of their survivorship experience than frequent PSA testing, and this may have implications for decision making about surveillance approaches to early prostate cancer. Finally, hormone therapy was associated with poorer physical well‐being, underlining the burden this treatment type carries for men with prostate cancer. Receiving RP or BT was predictive of men experiencing some cancer‐related distress; however, the reasons for this are unclear.
Importantly, it is clear that the course of adjustment for many men will not be linear. Ongoing assessment of function as well as QoL and psychological distress is needed into the medium and long term, with linked accessible support systems. In many settings, this will require the integration of primary and community care services as men leave the acute treatment system. To date, peer support has been a leading model of care in the community36, 37 and it seems likely that as prevalence and the health costs of prostate cancer increase, so too will the importance of peer support in survivorship care. Future research to develop and evaluate integrated long‐term survivorship care systems is needed.
Limitations of the current study include the use of a nonrepresentative sample and the extent of study attrition over time. Specifically, the cohort was more likely to be have been diagnosed with intermediate than advanced prostate cancer compared to all men diagnosed with prostate cancer in Queensland during the same period.20 This means that generalisability to the population should be approached cautiously and that we were unable to explore the effect of stage of illness on outcomes. As well, we did not have matched controls as a comparison group. As a further comment, masculinity is increasingly acknowledged as influential in men's response to prostate cancer.38 There is a need for future research to include masculinity as a potential mediator or moderator of adjustment outcomes that will be aided by the recent development of a context specific measure for this purpose.38 Study strengths include the high response rate, large sample, application of well validated and reliable measures, and long‐term (>5 years) follow‐up. In addition, the application of GMMs presents a new picture of posttreatment adjustment not previously presented, providing a more accurate picture of differences in individual change over time than the conventional growth model and the latent class growth analysis method while allowing also for nonlinear change to be described.
In conclusion, prostate cancer survivorship is a neglected area of research.39 Medium‐ to long‐term survivorship care plans for men with prostate cancer will need to consider the influence of age, partner status, and social disadvantage and comorbidities, as well as urinary, sexual, and bowel function, on men's QoL and psychological outcomes. A holistic approach that considers life course as well as medical treatment regimens is needed. For many men, the path of recovery and survivorship will not be linear, with the implication that regular assessment of progress towards optimal well‐being is needed over the medium to long term, with care plans adjusted to meet emerging issues.
ACKNOWLEDGEMENTS
This project was funded by the National Health and Medical Research Council (ID442301) and Cancer Council Queensland. We gratefully acknowledge the support of the Urological Society of Australia and New Zealand.
Chambers SK, Ng SK, Baade P. et al. Trajectories of quality of life, life satisfaction, and psychological adjustment after prostate cancer. Psycho‐Oncology. 2017;26:1576–1585. https://doi.org/10.1002/pon.4342
REFERENCES
- 1. Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide: IARC CancerBase No. 11. 2012: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed 11 July 2016.
- 2. Winter A, Sirri E, Jansen L, et al. Comparison of prostate cancer survival in Germany and the USA: can differences be attributed to differences in stage distributions? BJU Int. 2016. doi: 10.1111/bju.13537 [DOI] [PubMed] [Google Scholar]
- 3. Baade PD, Youlden DR, Chambers SK. When do I know I am cured? Using conditional estimates to provide better information about cancer survival prospects. Med J Aust. 2011;194(2):73. [DOI] [PubMed] [Google Scholar]
- 4. Klaff R, Rosell J, Varenhorst E, Sandblom G. The long‐term disease‐specific mortality of low‐risk localized prostate cancer: a prospective population‐based register study over two decades. Urology. 2016;91:77–82. [DOI] [PubMed] [Google Scholar]
- 5. Surbone A, Tralongo P. Categorization of cancer survivors: why we need it. J Clin Oncol. 2016;34(28):3372–3374. [DOI] [PubMed] [Google Scholar]
- 6. Potosky AL, Davis WW, Hoffman RM, et al. Five‐year outcomes after prostatectomy or radiotherapy for prostate cancer: the prostate cancer outcomes study. J Natl Cancer Inst. 2004;96(18):1358–1367. [DOI] [PubMed] [Google Scholar]
- 7. Punnen S, Cowan JE, Chan JM, Carroll PR, Cooperberg MR. Long‐term health‐related quality of life after primary treatment for localized prostate cancer: results from the CaPSURE registry. Eur Urol. 2015;68(4):600–608. [DOI] [PubMed] [Google Scholar]
- 8. Chambers SK, Zajdlewicz L, Youlden DR, Holland JC, Dunn J. The validity of the distress thermometer in prostate cancer populations. Psycho‐Oncol. 2014;23(2):195–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hinz A, Krauss O, Stolzenburg J‐U, Schwalenberg T, Michalski D, Schwarz R. Anxiety and depression in patients with prostate cancer and other urogenital cancer: a longitudinal study. Urol Oncol Semin Orig Investigations. 2009;27(4):367–372. [DOI] [PubMed] [Google Scholar]
- 10. Johansson E, Steineck G, Holmberg L, et al. Long‐term quality‐of‐life outcomes after radical prostatectomy or watchful waiting: the Scandinavian Prostate Cancer Group‐4 randomised trial. Lancet Oncol. 2011;12(9):891–899. [DOI] [PubMed] [Google Scholar]
- 11. Jongkamp VG, Roeloffzen EMA, Monninkhof EM, de Leeuw JRJ. Lycklama à Nijeholt AAM, van Vulpen M. Brachytherapy for prostate cancer does not influence long‐term depression rate. Brachytherapy. 2012;11(6):495–501. [DOI] [PubMed] [Google Scholar]
- 12. Korfage IJ, Essink‐Bot ML, Janssens ACJW, Schroder FH, de Koning HJ. Anxiety and depression after prostate cancer diagnosis and treatment: 5‐year follow‐up. Br J Cancer. 2006;94(8):1093–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stull DE, Wiklund I, Gale R, Capkun‐Niggli G, Houghton K, Jones P. Application of latent growth and growth mixture modeling to identify and characterize differential responders to treatment for COPD. Contemp Clin Trials. 2011;32(6):818–828. [DOI] [PubMed] [Google Scholar]
- 14. Land KC, McCall PL, Nagin DS. A comparison of Poisson, negative binomial, and semiparametric mixed Poisson regression models: with empirical applications to criminal careers data. Soc Meth Res. 1996;24(4):387–442. [Google Scholar]
- 15. Mplus Users Guide [computer program]. Version 6.12. Los Angeles, CA: Muthen & Muthen; 2010.
- 16. Dunn J, Ng S, Holland J, et al. Trajectories of psychological distress after colorectal cancer. Psycho‐Oncol. 2013;22:1759–1765. [DOI] [PubMed] [Google Scholar]
- 17. Dunn J, Ng SK, Breitbart W, et al. Health‐related quality of life and life satisfaction in colorectal cancer survivors: trajectories of adjustment. Health Qual Life Outcomes. 2013;11(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baade PD, Aitken JF, Ferguson M, Gardiner RA, Chambers SK. Diagnostic and treatment pathways for men with prostate cancer in Queensland: investigating spatial and demographic inequalities. BMC Cancer. 2010;10(1):452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baade PD, Gardiner RA, Ferguson M, et al. Factors associated with diagnostic and treatment intervals for prostate cancer in Queensland, Australia: a large cohort study. Cancer Causes and Control. 2012;23(4):1–10. [DOI] [PubMed] [Google Scholar]
- 20. Baade PD, Youlden DR, Gardiner RA, et al. Factors associated with treatment received by men diagnosed with prostate cancer in Queensland Australia. BJU Int. 2012;110(11b):E712–E719. [DOI] [PubMed] [Google Scholar]
- 21. Medical Outcomes Trust and Quality Metric Incorporated . SF‐36: SF‐36v2TM Health Survey; (IQOLA SF36v2 Standard, English (Australia), 7/03). Lincoln, RI: Medical Outcomes Trust and Quality Metric Incorporated, Health Assessment Lab; 1992. 2003 [Google Scholar]
- 22. Wei JT, Dunn RL, Litwin MS, Sandler HM, Sanda MG. Development and validation of the expanded prostate cancer index composite (EPIC) for comprehensive assessment of health‐related quality of life in men with prostate cancer. Urology. 2000;56(6):899–905. [DOI] [PubMed] [Google Scholar]
- 23. Diener E, Emmons RA, Larsen RJ, Griffin S. The Satisfaction With Life Scale. J Pers Assess. 1985;49(1):71–75. [DOI] [PubMed] [Google Scholar]
- 24. Weiss DS, Marmar CR. The Impact of Events Scale ‐ Revised In: Wilson JP, Keane TM, eds. Assessing Psychological Trauma and PTSD. New York, NY: The Guildford Press; 1997:399–411. [Google Scholar]
- 25. Roth A, Nelson CJ, Rosenfeld B, et al. Assessing anxiety in men with prostate cancer: further data on the reliability and validity of the Memorial Anxiety Scale for Prostate Cancer (MAX‐PC). Psychosomatics. 2006;47(4):340–347. [DOI] [PubMed] [Google Scholar]
- 26. Roth AJ, Rosenfeld B, Kornblith AB, et al. The memorial anxiety scale for prostate cancer: validation of a new scale to measure anxiety in men with prostate cancer. Cancer. 2003;97(11):2910–2918. [DOI] [PubMed] [Google Scholar]
- 27. Muthén B. Latent variable analysis In: The Sage Handbook of Quantitative Methodology for the Social Sciences. Thousand Oaks, CA: Sage Publications; 2004:345–368. [Google Scholar]
- 28. Infurna FJ, Luthar SS. Resilience to major life stressors is not as common as thought. Perspect Psychol Sci. 2016;11(2):175–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reinecke J, Seddig D. Growth mixture models in longitudinal research. AStA Adv Stat Anal. 2011;95(4):415–434. [Google Scholar]
- 30. Jung T, Wickrama K. An introduction to latent class growth analysis and growth mixture modeling. Soc Personal Psychol Compass. 2008;2(1):302–317. [Google Scholar]
- 31. Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons; 2004;81. [Google Scholar]
- 32. Curran PJ, Obeidat K, Losardo D. Twelve Frequently asked questions about growth curve modeling. J Cogn Dev. 2010;11(2):121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chambers SK, Lowe A, Hyde MK, et al. Defining young in the context of prostate cancer. Am J Mens Health. 2015;9(2):103–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Song L, Ji Y, Nielsen ME. Quality of life and health status among prostate cancer survivors and noncancer population controls. Urology. 2014;83(3):658–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song L, Mishel M, Bensen JT, et al. How does health literacy affect quality of life among men with newly diagnosed clinically localized prostate cancer? Cancer. 2012;118(15):3842–3851. [DOI] [PubMed] [Google Scholar]
- 36. Dunn J, Steginga S, Rosoman N, Millichap D. A review of peer support in the context of cancer. J Psychosoc Oncol. 2003;21(2):55–67. [Google Scholar]
- 37. Steginga SK, Pinnock C, Gardner M, Gardiner R, Dunn J. Evaluating peer support for prostate cancer: the prostate cancer peer support inventory. BJU Int. 2005;95(1):46–50. [DOI] [PubMed] [Google Scholar]
- 38. Chambers S, Hyde MK, Zajdlewicz L, et al. Measuring masculinity in the context of chronic disease. Psychol Men Masc. 2015;17(3):228–242. [Google Scholar]
- 39. Chambers SK, Scuffham PA, Baade PD, et al. Advancing prostate cancer survivorship research in Australia. Cancer Forum. 2015;39(3):204–209. [Google Scholar]


