Abstract
AMP-activated protein kinase (AMPK) is known as a pivotal regulator of cellular metabolism. Mounting evidences have demonstrated that AMPK activation exerts tumor suppressive activity on leukemia cells. The present study reported that adenine, an AMPK activator, triggers cell cycle arrest and autophagy of human chronic myelogenous leukemia K562 cells consequently suppressing cell viability. The present findings revealed that adenine treatment (4.0–8.0 mM) significantly inhibited the viability of K562 cells to 69.3±2.5% (24 h) and 53.4±2.1% (48 h) of the control. Flow cytometric analysis revealed that there was a significant accumulation in G2/M phase, but not sub-G1 phase K562 cells following exposure to adenine. Additional investigation demonstrated that adenine treatments significantly increased the number of acidic vesicular organelles and the level of autophagosomal microtubule associated protein 1 light chain 3 α (LC3) marker. By contrast, cleavage of caspase-9, caspase-3 and poly-ADP-ribose polymerase was insignificantly affected in K562 cells following adenine treatment. In K562 cells, adenine was able to markedly promote the phosphorylation of AMPKα and suppress the phosphorylation of mammalian target of rapamycin (mTOR), a downstream target of AMPK. In addition, inhibiting AMPK phosphorylation using dorsomorphin restored mTOR phosphorylation, inhibited the accumulation of LC3 and significantly recovered the suppressed cell viability in response to adenine. Taken together, the present results demonstrated that adenine induced G2/M phase arrest and autophagic cell death, consequently suppressing the viability of K562 cells, which may attribute to the AMPK activation triggered by adenine. These findings provide evidence that adenine may be beneficial to chronic myelogenous leukemia therapy by suppressing excessive cell proliferation.
Keywords: adenine, AMP-activated protein kinase, autophagy, cell cycle arrest, chronic myelogenous leukaemia K562 cells
Introduction
Chronic myelogenous leukemia (CML) is a chronic myeloproliferative disorder, which causes uncontrolled growth of immature myeloid cells (1). The BCR RhoGEF and GTPase activating protein (BCR)-ABL proto-oncogene 1 non-receptor tyrosine kinase (ABL) gene rearrangement is the main characteristic of CML, which expresses the oncogenic fusion protein BCR-ABL (2). BCR-ABL is a constitutively active tyrosine kinase, which activates multiple signaling pathways and consequently promotes malignant transformation, including uncontrolled cell proliferation (3), abnormal cell adhesion (4), and resistance to typical apoptotic inducer anti-leukemic drugs (5,6). Thus, formation of the BCR-ABL fusion gene serves an essential role in the pathogenesis of CML (7). Previously, imatinib, a specific ABL kinase inhibitor, was established as the standard treatment for CML (8).
In addition to targeting BCR-ABL kinase, previous studies have revealed that several pathways are important for CML cell survival, which may be potential targets for developing novel anti-leukemia drugs (9,10). Among these pathways, AMP-activated protein kinase (AMPK) signaling has been reported to possess anti-leukemia activity (11). AMPK serves an important role in energy metabolism in response to changes in cellular fuel levels (12). Furthermore, mounting evidences have suggested that AMPK could be a target for tumor prevention and treatment (7). Previous studies have revealed that AMPK activators exhibit anti-leukemia effects in CML cells by triggering apoptosis and autophagy (3,4). Thus, the identification and characterization of AMPK activators is important, and beneficial for the development of potential anti-leukemia drugs.
The present study aimed to investigate whether adenine, a purine compound that induces AMPK activation, exhibits anti-leukemia effects on human CML cells. Furthermore, the underlying mechanism, with emphasis on AMPK signaling was evaluated. The results revealed that adenine suppressed cell viability of K562 cells and induced accumulation of G0/G1 phase cells. In parallel, it was observed that adenine triggered K562 cells autophagy. Finally, it was demonstrated that suppressed cell viability, accumulation of G0/G1 phase cells and induction of autophagy were associated with AMPK activation in response to adenine.
Materials and methods
Reagents
All chemicals were obtained from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany) unless otherwise specified. RPMI-1640 medium and fetal bovine serum (FBS) were purchased from Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA). Antibodies against β-actin, caspase-3, caspase-8, phosphorylated (p)-AMPK (T172; cat. no. 2535), AMPK (cat. no. 2532), poly-ADP-ribose polymerase (PARP) (cat. no. 9532), cell division cycle (Cdc)25 (cat. no. 3652), cyclin-dependent kinase (CDK)2 (cat. no. 2546), CDK4 (cat. no. 12790), CDK6 (cat. no. 13331), cyclin B (cat. no. 4135), cyclin E (cat. no. 20808), Wee1-like protein kinase (Wee1) (cat. no. 13084), autophagy protein (Atg)5 (cat. no. 12994), beclin-1 (cat. no. 3495) and microtubule associated protein 1 light chain 3 α (LC3) (cat. no. 3868) were purchased from Cell Signaling Technologies, Inc. (Danvers, MA, USA). Anti-human glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (cat. no. ab9485), horseradish peroxidase-conjugated anti-mouse IgG (cat. no. ab6789) and anti-rabbit IgG (cat. no. ab6721) antibodies were purchased from Abcam (Cambridge, UK).
Cell culture and experimental treatments
The human CML K562 cell line (CCL-243™) was obtained from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI-1640 medium supplemented with 10% FBS at 37°C in a humidified atmosphere with 5% CO2. Cells at the density of 5×105 cells/ml were collected and were passaged twice prior to being subcultured T-75 flasks (Corning Incorporated, Corning, NY, USA) for subsequent treatments.
For treatments, cells were seeded in 6-well plates at an initial density of 1×105 cells/ml and grown to ~80% confluence at 37°C. Treatments were performed by incubating cells with various concentrations of adenine (0.5, 1.0, 2.0, 4.0 or 8.0 mM) in serum-free RPMI-1640 for 16 h. AMPK inhibition was performed by pretreating cells with 5 µM dorsomorphin (cat. no. P5499; Sigma-Aldrich; Merck KGaA) for 2 h, and then incubating the pretreated cells with 4.0 mM adenine for 24 h or 48 h. Following treatment, the cells were washed with PBS (25 mM sodium phosphate, 150 mM NaCl, pH 7.2) and collected for subsequent analyses.
Cell viability assay
Cell viability was assessed using an MTT assay protocol as previously described (13) in the absence or presence of adenine. After 24 or 48 h treatments, cells were harvested, and then incubated with MTT (0.5 mg/ml) at 37°C for 4 h. The cell viability was directly proportional to the production of formazan, which was dissolved in isopropanol and determined by measuring the absorbance at 570 nm using a microplate reader (SpectraMAX 360 pc; Molecular Devices, LCC, Sunnyvale, CA, USA).
Cell cycle distribution analysis
Cells were starved for 16 h in serum-free medium, and then cultured in fresh serum-containing medium at 37°C for 24 h to allow cell-cycle progression. Following different treatments, cells were fixed and analyzed by flow cytometry to determine cell cycle distribution. At the end of each treatment, cells were collected, fixed with 1 ml of ice-cold 70% ethanol on ice for 30 min, incubated at −20°C for 24 h and centrifuged at 380 × g at 4°C for 5 min. Cell pellets were reacted with l ml cold staining solution containing 20 µg/ml propidium iodide, 20 µg/ml RNase A and 1% Triton X-100, incubated at 4°C for 15 min in the dark, and then analyzed using a FACS Calibur system with CellQuest™ software (version 2.0) (both BD Biosciences, Franklin Lakes, NJ, USA). Representative results were acquired from three independent experiments.
Western blot analysis
Cells were washed with PBS and then incubated with lysis buffer (50 mM Tris-HCl, pH 7.5, 1% Nonidet P-40, 1 mM phenylmethylsulfonyl fluoride and 1 mM NaF) containing complete protease inhibitor cocktail (Roche Applied Science, Mannheim, Germany) for 1 h at 4°C. The resulting supernatants were collected for protein ßquantitation using a bicinchoninic acid kit (Pierce; Thermo Fisher Scientific, Inc.). Crude proteins (20 µg) were subjected to 12.5% SDS-PAGE, transferred to a nitrocellulose membrane (EMD Millipore, Billerica, MA, USA) and then incubated with 5% w/v skimmed milk/PBS at room temperature for 1 h to block nonspecific binding. The blocked membrane was then incubated with primary antibodies (1:1,000 dilution) at 25°C for 2 h, followed by incubation with peroxidase-conjugated anti-IgG secondary antibodies (1:2,000 dilution) at 25°C for 1 h. Detailed information for antibodies were described in the reagents. The blocked membrane without incubation with primary antibodies was also incubated with secondary antibodies for specificity test, and a nonsignificant signal was detected as the control. Signal development was performed using an enhanced chemiluminescence reagent (EMD Millipore). Luminescent signals were acquired and quantified using an image analysis system (LAS-4000 with Image Reader LAS-4000 version 2.1, Fuji, Tokyo, Japan). GAPDH was used as an internal control.
Quantification of acidic vesicular organelles (AVOs) using acridine orange staining and flow cytometric analysis
Cells (1×104) were incubated with acridine orange at 25°C for 17 min, harvested with trypsin-EDTA, and then collected in phenol red-free growth medium (Thermo Fisher Scientific, Inc.). Green (510–530 nm) and red (650 nm) fluorescence emission from cells illuminated with blue (488 nm) excitation light was analyzed using FACS Calibur system using CellQuest software as mentioned above. With acridine orange staining, bright green and faint red fluorescence was observed in the cytoplasm and nucleolus, respectively, and bright red fluorescence was observed in acidic compartments (2,14). Since the intensity of the red fluorescence is proportional to the degree of acidity, the volume of the cellular acidic compartment could be quantified (15).
Statistical analysis
Data are expressed as the mean ± standard error of the mean following three independent experiments. Statistical analysis was performed by using SigmaStat (version 4.0, Systat Software, Inc, San Jose, CA, USA). Statistical significance analysis was determined using one-way analysis of variance followed by Dunnett's test for multiple comparisons with the control. P<0.05 was considered to indicate a statistically significant difference.
Results
Adenine suppresses the viability of human CML K562 cells
To investigate the effect of adenine on human CML K562 cells, cell viability was determined using MTT assay. As presented in Fig. 1, for 24 h treatments, the viability of K562 cells was significantly reduced to 81.5±2.1, 75.4±2.2 and 69.3±2.5% of the control in response to 2, 4 and 8 mM adenine, respectively (all P<0.05). For 48 h treatments, the cell viability was further reduced to 61.3±2.3 and 53.4±3.1% of the control in response to 4 and 8 mM adenine, respectively (both P<0.01). These findings revealed that adenine at concentrations of 2, 4 and 8 mM significantly inhibited the viability of K562 cells.
Figure 1.
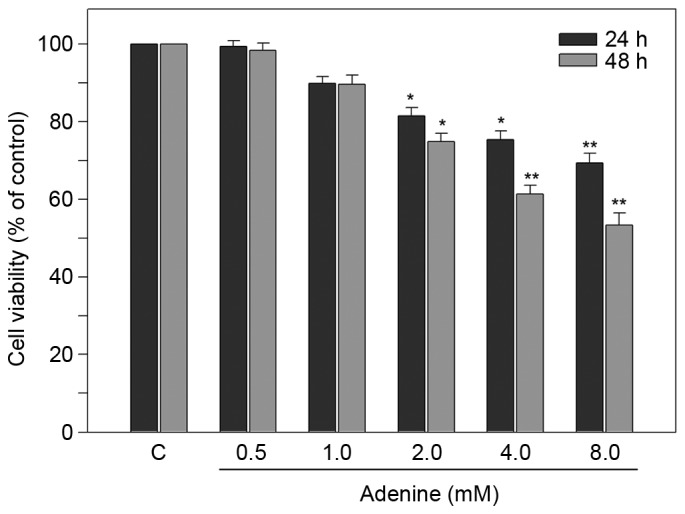
Adenine suppresses the viability of K562 cells. Cells were treated with various concentrations of adenine (0.5, 1.0, 2.0, 4.0 or 8.0 mM) for 24 or 48 h, and then subjected to a cell viability assay. Data are expressed as the mean ± standard error of the mean following three independent experiments. *P<0.05 and **P<0.01 as compared with the untreated control. C, control.
Adenine induces G2/M cell cycle arrest and alters cell cycle regulators in human CML K562 cells
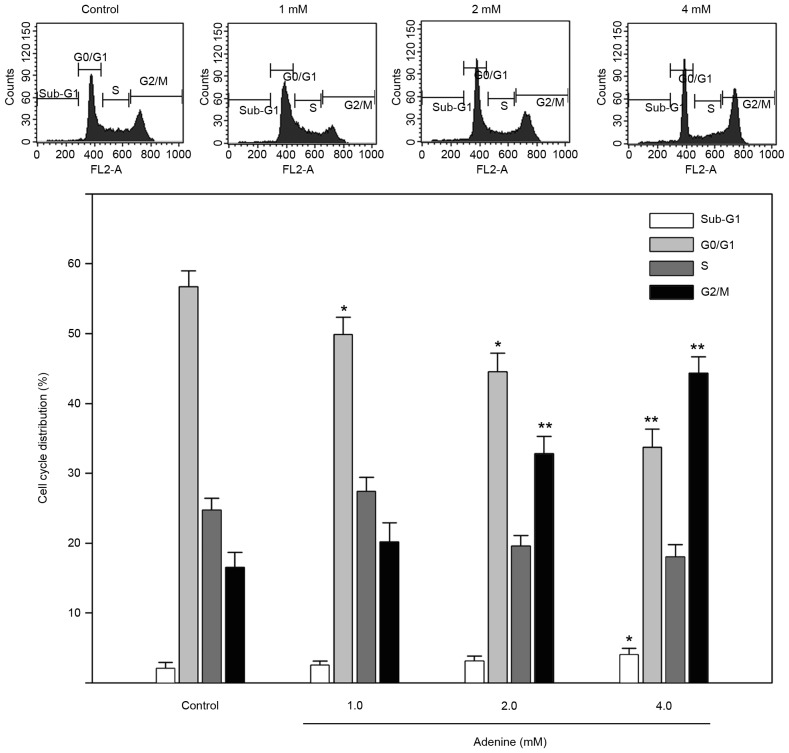
To explore the inhibited cell viability in response to adenine, the cell cycle distribution of K562 cells treated with adenine was determined using flow cytometric analysis. As presented in Fig. 2, the sub-G1 phase ratio was increased by 4.1±0.7% in K562 cells treated with 4 mM adenine compared with the control group (P<0.05). In addition, the G2/M phase ratio was significantly increased in K562 cells treated with 2 mM (32.1±2.7%) and 4 mM (43.8±2.1%) adenine compared with that in the untreated control group (both P<0.01). The G0/G1 phase ratio was significantly decreased in K562 cells treated with 2 mM (44.6±2.7%) and 4 mM (33.8±2.4%) adenine compared with that in untreated cells (both P<0.05).
Figure 2.
Effects of adenine on the cell cycle distribution of K562 cells. Cells were treated with 1.0, 2.0 or 4.0 mM adenine for 24 h, and then subjected to flow cytometric analysis for determining cell cycle phases. The ratio of each phase is presented as a percentage. Data are expressed as the mean ± standard error of the mean following three independent experiments. *P<0.05 and **P<0.01 as compared with the untreated control.
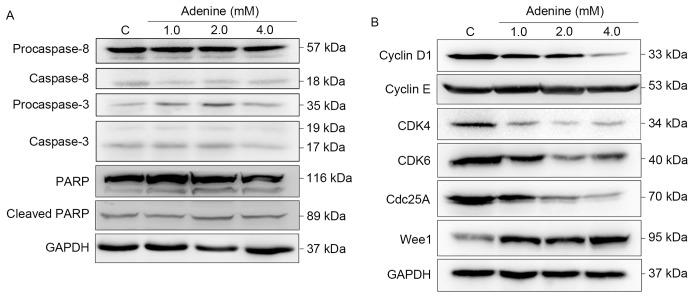
Since cell viability and cell cycle distribution were altered following adenine treatment, the effects of adenine on caspase signaling cascades, and cell cycle regulators were then investigated. As presented in Fig. 3A, cleavage of caspase-8, caspase-3 and PARP was insignificantly affected by adenine treatments (1.0, 2.0 or 4.0 mM). Regarding cell cycle regulators, the protein expression levels of Cyclin D1, CDK4, CDK6 and Cdc25A were markedly decreased in response to adenine treatments (Fig. 3B). By contrast, the level of Wee1 was elevated upon adenine stimulation. Notably, Cyclin E protein expression was insignificantly affected by adenine. Taken together, these findings suggest that adenine treatment induces G2/M cell cycle arrest without involvement of the activation of caspase signaling cascades and consequent apoptosis of K562 cells.
Figure 3.
Adenine inhibits cell cycle regulators but not caspase effectors. Cells were treated with 1.0, 2.0 and 4.0 mM adenine for 24 h, and then lysed for determination of protein expression levels using western blot analysis. GAPDH was used as an internal control. The apparent molecular weights of the detected proteins are indicated. PARP, poly-ADP-ribose polymerase; CDK, cyclin-dependent kinase; Cdc25A, cell division cycle 25A; Wee1, Wee1-like protein kinase; C, control.
Adenine induces autophagy of human CML K562 cells
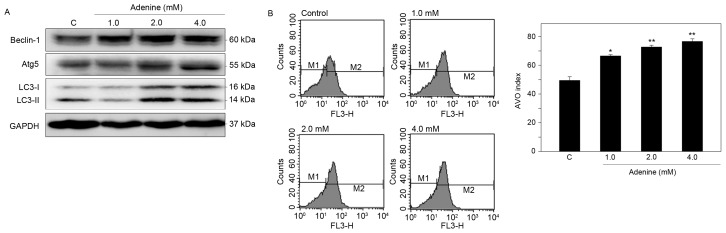
To determine whether adenine suppresses cell viability via autophagic cell death, induction of autophagy in K562 cells was then examined. Western blot analysis demonstrated that adenine markedly upregulated the protein expression of Beclin-1 and Atg5, and promoted the conversion of LC3-I to LC3-II in the cells (Fig. 4A). In addition, using acridine orange staining and flow cytometric quantification, adenine was identified to significantly increase the number of AVOs in K562 cells in a dose-dependent manner (Fig. 4B). These findings suggest that adenine treatment promotes the formation of autophagosomes in K562 cells.
Figure 4.
Adenine induces autophagy in K562 cells. Cells were treated with 1.0, 2.0 or 4.0 mM adenine for 24 h, and then subjected to (A) western blot analysis for determination of protein levels, or (B) acridine orange staining for detection of AVOs. GAPDH was used as internal control. The apparent molecular weights of the detected proteins are indicated. Quantitative data are expressed as the mean ± standard error of the mean following three independent experiments. *P<0.05 and **P<0.01 as compared with the untreated control. AVO, acidic vesicular organelle; Atg5, autophagy protein 5; LC3, microtubule associated protein 1 light chain 3 α; C, control.
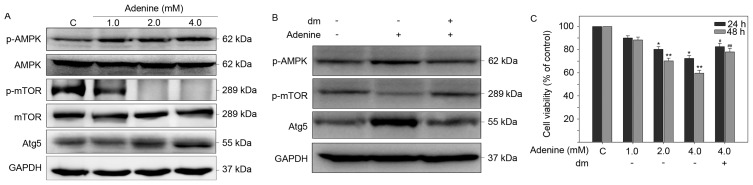
Adenine induces the phosphorylation of AMPKα, contributing to inhibition of mTOR and autophagic signaling cascades in K562 cells
Adenine has been reported to exert antitumoral activity on human renal carcinoma 786-O cells via AMPK activation (16). Thus, the roles of AMPK signaling in autophagic cell death of K562 cells in response to adenine were investigated. As presented in Fig. 5A, adenine markedly enhance the phosphorylation of AMPKα at T172, reduced the phosphorylation of mTOR at S2448 and elevated the protein expression level of autophagic marker Atg5 compared with untreated cells. By contrast, pretreatment with the AMPK inhibitor dorsomorphin (dm) reversed the expression changes to p-AMPKa, p-mTOR and Atg5 in K562 cells following exposure to adenine (Fig. 5B). In addition, pretreatment with dm significantly restored the viability of K562 cells suppressed by 4.0 mM adenine (Fig. 5C). Collectively, these findings suggest that the AMPK/mTOR signaling pathway is involved in adenine-induced autophagy and consequent viability inhibition of K562 cells.
Figure 5.
AMPK activation is involved in autophagy and suppression of viability of K562 cells in response to adenine. Cells were (A) treated with adenine alone or (B) pretreated with AMPK inhibitor dm (5 µM) for 2 h followed by treatment with adenine for 24 h, and then subjected to western blot analysis. GAPDH was used as internal control. The apparent molecular weights of the detected proteins are indicated. Quantitative data are expressed as the mean ± standard error of the mean following three independent experiments. (C) A cell viability assay was performed to investigate the effects of dm on K564 cells. *P<0.05 and **P<0.01 as compared with the untreated control; #P<0.05 and ##P<0.01 as compared with the 4.0 mM adenine treatment group. AMPK, AMP-activated protein kinase; p, phosphorylated; mTOR, mechanistic target of rapamycin; Atg5, autophagy protein 5; dm, dorsomorphin; C, control.
Discussion
Clinically, the Philadelphia chromosome exists in ~95% of adults with CML and expresses the BCR-ABL fusion protein with uncontrolled tyrosine kinase activity, resulting in abnormal regulation of downstream signaling pathways (17). The aberrant activation of signaling pathways, including the RAS/mitogen activated protein kinase kinase/extracellular signal-related kinase and phosphoinositide 3-kinase/RAC-alpha serine/threonine-protein kinase pathways, may promote cell proliferation, diminish cell apoptosis or contribute to growth factor independence of cancer cells (18,19). Therefore, tyrosine kinase inhibitors targeting the BCR-ABL fusion protein, including imatinib, dasatinib and nilotinib, are used for CML treatment, and have demonstrated improved outcomes in patients with CML (20). However, high relapse rates, drug resistance and high mortality rates associated with transplantation remain challenges for CML treatment (21).
Previous studies have revealed that AMPK activators can suppress several types of tumors, including pancreatic, bladder and prostate cancer, through AMPK-dependent apoptosis (19–21). In addition, AMPK has been reported as a potential target that regulates various signaling pathways, subsequently exhibiting antileukemia activity (22). Among the signaling pathways regulated by AMPK, mTOR is known to perform a central role in antitumor activity (23). Recently, several compounds, including metformin and myrtucommulone A, have been reported to exert antileukemia activity via activation of the AMPK/mTOR signaling pathway (24,25). Similarly, the present results revealed that adenine suppressed the viability of K562 cells through activation of the AMPK/mTOR signaling pathway, resulting in G2/M phase arrest and autophagic cell death.
Autophagy serves an important role in regulating cellular physiology, removing senescent organelles and degrading long-lived proteins (26,27). In starved cells, the fatty acids and amino acids produced by hydrolysis of lipids, and proteins in autophagolysosomes provide energy to maintain cell survival (28). However, elongated autophagy is proposed to trigger autophagic caspase-independent type II programmed cell death (29). Thus, the roles of autophagy in sustaining or killing cancer cells are complicated. Although cytotoxicity of antitumor drugs is diminished by autophagy to a certain extent (30), reduced expression of autophagic genes, including Atg4, Atg5 and Atg7, has been reported to promote tumor formation in genetically-engineered mice (31,32). Collectively, the findings suggest that autophagy possesses tumor suppressive activity. In the present study, it was demonstrated that adenine can trigger autophagic cell death, but not apoptosis, consequently suppressing the viability of K562 cells. Thus the results of the current study indicate that adenine may serve as a potential anti-leukemia agent.
In conclusion, the present study demonstrated that adenine treatment significantly suppresses the viability of CML K562 cells through activation of the AMPK/mTOR pathway and synergistic induction of G2/M phase arrest, and autophagy signaling. Thus, adenine represents a promising effective antiproliferative agent against human leukemia cells.
References
- 1.Clarkson B, Strife A, Wisniewski D, Lambek CL, Liu C. Chronic myelogenous leukemia as a paradigm of early cancer and possible curative strategies. Leukemia. 2003;17:1211–1262. doi: 10.1038/sj.leu.2402912. [DOI] [PubMed] [Google Scholar]
- 2.Traganos F, Darzynkiewicz Z. Lysosomal proton pump activity: Supravital cell staining with acridine orange differentiates leukocyte subpopulations. Methods Cell Biol. 1994;41:185–194. doi: 10.1016/S0091-679X(08)61717-3. [DOI] [PubMed] [Google Scholar]
- 3.Vakana E, Altman JK, Glaser H, Donato NJ, Platanias LC. Antileukemic effects of AMPK activators on BCR-ABL-expressing cells. Blood. 2011;118:6399–6402. doi: 10.1182/blood-2011-01-332783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sujobert P, Poulain L, Paubelle E, Zylbersztejn F, Grenier A, Lambert M, Townsend EC, Brusq JM, Nicodeme E, Decrooqc J, et al. Co-activation of AMPK and mTORC1 induces cytotoxicity in acute myeloid leukemia. Cell Rep. 2015;11:1446–1457. doi: 10.1016/j.celrep.2015.04.063. [DOI] [PubMed] [Google Scholar]
- 5.Bedi A, Zehnbauer BA, Barber JP, Sharkis SJ, Jones RJ. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood. 1994;83:2038–2044. [PubMed] [Google Scholar]
- 6.Klionsky DJ, Meijer AJ, Codogno P. Autophagy and p70S6 kinase. Autophagy. 2005;1:59–61. doi: 10.4161/auto.1.1.1536. [DOI] [PubMed] [Google Scholar]
- 7.Kim I, He YY. Targeting the AMP-activated protein kinase for cancer prevention and therapy. Front Oncol. 2013;3:175. doi: 10.3389/fonc.2013.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vakana E, Platanias LC. AMPK in BCR-ABL expressing leukemias. Regulatory effects and therapeutic implications. Oncotarget. 2011;2:1322–1328. doi: 10.18632/oncotarget.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Druker B, Okuda K, Matulonis U, Salgia R, Roberts T, Griffin JD. Tyrosine phosphorylation of rasGAP and associated proteins in chronic myelogenous leukemia cell lines. Blood. 1992;79:2215–2220. [PubMed] [Google Scholar]
- 10.Gotoh A, Miyazawa K, Ohyashiki K, Tauchi T, Boswell HS, Broxmeyer HE, Toyama K. Tyrosine phosphorylation and activation of focal adhesion kinase (p125FAK) by BCR-ABL oncoprotein. Exp Hematol. 1995;23:1153–1159. [PubMed] [Google Scholar]
- 11.Fernandes A, Azevedo MM, Pereira O, Sampaio-Marques B, Paiva A, Correia-Neves M, Castro I, Ludovico P. Proteolytic systems and AMP-activated protein kinase are critical targets of acute myeloid leukemia therapeutic approaches. Oncotarget. 2015;6:31428–31440. doi: 10.18632/oncotarget.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 14.Stankiewicz M, Jonas W, Hadas E, Cabaj W, Douch PG. Supravital staining of eosinophils. Int J Parasitol. 1996;26:445–446. doi: 10.1016/0020-7519(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 15.Paglin S, Hollister T, Delohery T, Hackett N, McMahill M, Sphicas E, Domingo D, Yahalom J. A novel response of cancer cells to radiation involves autophagy and formation of acidic vesicles. Cancer Res. 2001;61:439–444. [PubMed] [Google Scholar]
- 16.Hsu CY, Lin CH, Lin JT, Cheng YF, Chen HM, Kao SH. Purine analogue ENERGI-F706 induces apoptosis of 786-O renal carcinoma cells via 5′-adenosine monophosphate-activated protein kinase activation. Mol Med Rep. 2015;12:4566–4571. doi: 10.3892/mmr.2015.3906. [DOI] [PubMed] [Google Scholar]
- 17.Sattler M, Griffin JD. Molecular mechanisms of transformation by the BCR-ABL oncogene. Semin Hematol. 2003;40(2 Suppl 2):S4–S10. doi: 10.1053/shem.2003.50034. [DOI] [PubMed] [Google Scholar]
- 18.Skorski T, Kanakaraj P, Nieborowska-Skorska M, Ratajczak MZ, Wen SC, Zon G, Gewirtz AM, Perussia B, Calabretta B. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood. 1995;86:726–736. [PubMed] [Google Scholar]
- 19.Cortez D, Reuther G, Pendergast AM. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene. 1997;15:2333–2342. doi: 10.1038/sj.onc.1201400. [DOI] [PubMed] [Google Scholar]
- 20.Mace ML, Dahl J, Jabbour EJ. Which tyrosine-kinase inhibitor to use first in chronic phase chronic myelogenous leukemia? Expert Opin Pharmacother. 2015;16:999–1007. doi: 10.1517/14656566.2015.1031107. [DOI] [PubMed] [Google Scholar]
- 21.Adekola K, Popat U, Ciurea SO. An update on allogeneic hematopoietic progenitor cell transplantation for myeloproliferative neoplasms in the era of tyrosine kinase inhibitors. Bone Marrow Transplant. 2014;49:1352–1359. doi: 10.1038/bmt.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Karnevi E, Said K, Andersson R, Rosendahl AH. Metformin-mediated growth inhibition involves suppression of the IGF-I receptor signalling pathway in human pancreatic cancer cells. BMC Cancer. 2013;13:235. doi: 10.1186/1471-2407-13-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng QY, Jin FS, Yao C, Zhang T, Zhang GH, Ai X. Ursolic acid-induced AMP-activated protein kinase (AMPK) activation contributes to growth inhibition and apoptosis in human bladder cancer T24 cells. Biochem Biophys Res Commun. 2012;419:741–747. doi: 10.1016/j.bbrc.2012.02.093. [DOI] [PubMed] [Google Scholar]
- 24.Sauer H, Engel S, Milosevic N, Sharifpanah F, Wartenberg M. Activation of AMP-kinase by AICAR induces apoptosis of DU-145 prostate cancer cells through generation of reactive oxygen species and activation of c-Jun N-terminal kinase. Int J Oncol. 2012;40:501–508. doi: 10.3892/ijo.2011.1230. [DOI] [PubMed] [Google Scholar]
- 25.Borthakur G, Duvvuri S, Ruvolo V, Tripathi DN, Piya S, Burks J, Jacamo R, Kojima K, Ruvolo P, Fueyo-Margareto J, et al. MDM2 inhibitor, nutlin 3a, induces p53 dependent autophagy in acute leukemia by AMP kinase activation. PLoS One. 2015;10:e0139254. doi: 10.1371/journal.pone.0139254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine B, Kroemer G. Autophagy in aging, disease and death: The true identity of a cell death impostor. Cell Death Differ. 2009;16:1–2. doi: 10.1038/cdd.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Settembre C, Fraldi A, Medina DL, Ballabio A. Signals from the lysosome: A control centre for cellular clearance and energy metabolism. Nat Rev Mol Cell Biol. 2013;14:283–296. doi: 10.1038/nrm3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23:2891–2906. doi: 10.1038/sj.onc.1207521. [DOI] [PubMed] [Google Scholar]
- 30.Aredia F, Scovassi AI. Manipulation of autophagy in cancer cells: An innovative strategy to fight drug resistance. Future Med Chem. 2013;5:1009–1021. doi: 10.4155/fmc.13.85. [DOI] [PubMed] [Google Scholar]
- 31.Liang XH, Jackson S, Seaman M, Brown K, Kempkes B, Hibshoosh H, Levine B. Induction of autophagy and inhibition of tumorigenesis by beclin 1. Nature. 1999;402:672–676. doi: 10.1038/45257. [DOI] [PubMed] [Google Scholar]
- 32.Yue Z, Jin S, Yang C, Levine AJ, Heintz N. Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci USA. 2003;100:15077–15082. doi: 10.1073/pnas.2436255100. [DOI] [PMC free article] [PubMed] [Google Scholar]