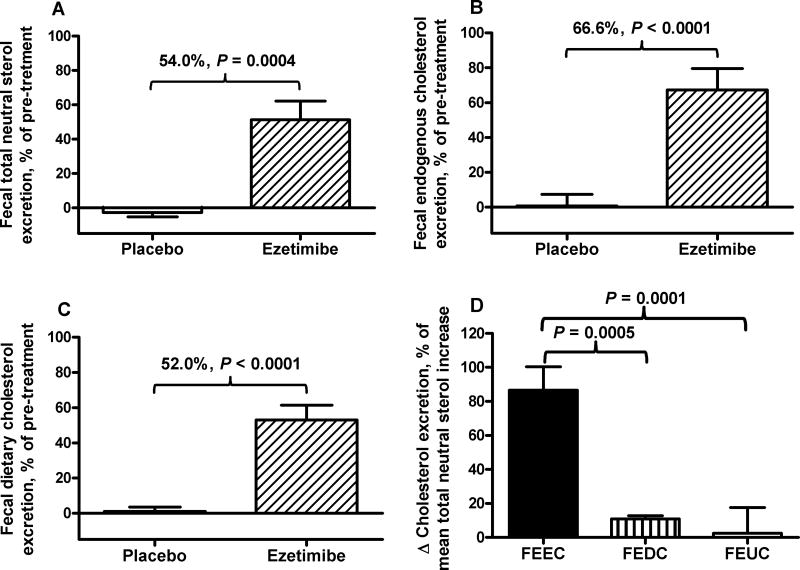
Fig. 2. Effects of ezetimibe on fecal excretion of total (A), endogenous (B), and dietary cholesterol (C), and relative contributions to fecal excretion of total neutral sterols by ezetimibe (D).
Fecal excretion of total and endogenous cholesterol was determined in subjects receiving 10 mg/day ezetimibe (n=12) or placebo (n=12) for 6 weeks, as described in Methods. Fecal excretion of unabsorbed dietary cholesterol and unlabeled cholesterol were calculated as described in Methods. Results of A, B, and C are expressed as percent change relative to pre-treatment in each group. Treatment effects and the P values are shown above the bars of placebo and ezetimibe. Results of D are increases of fecal excretion of endogenous cholesterol (FEEC), of dietary cholesterol excretion (FEDC), and of unlabeled cholesterol (FEUC), as percent of the mean increase in fecal excretion of total neutral sterols by ezetimibe.