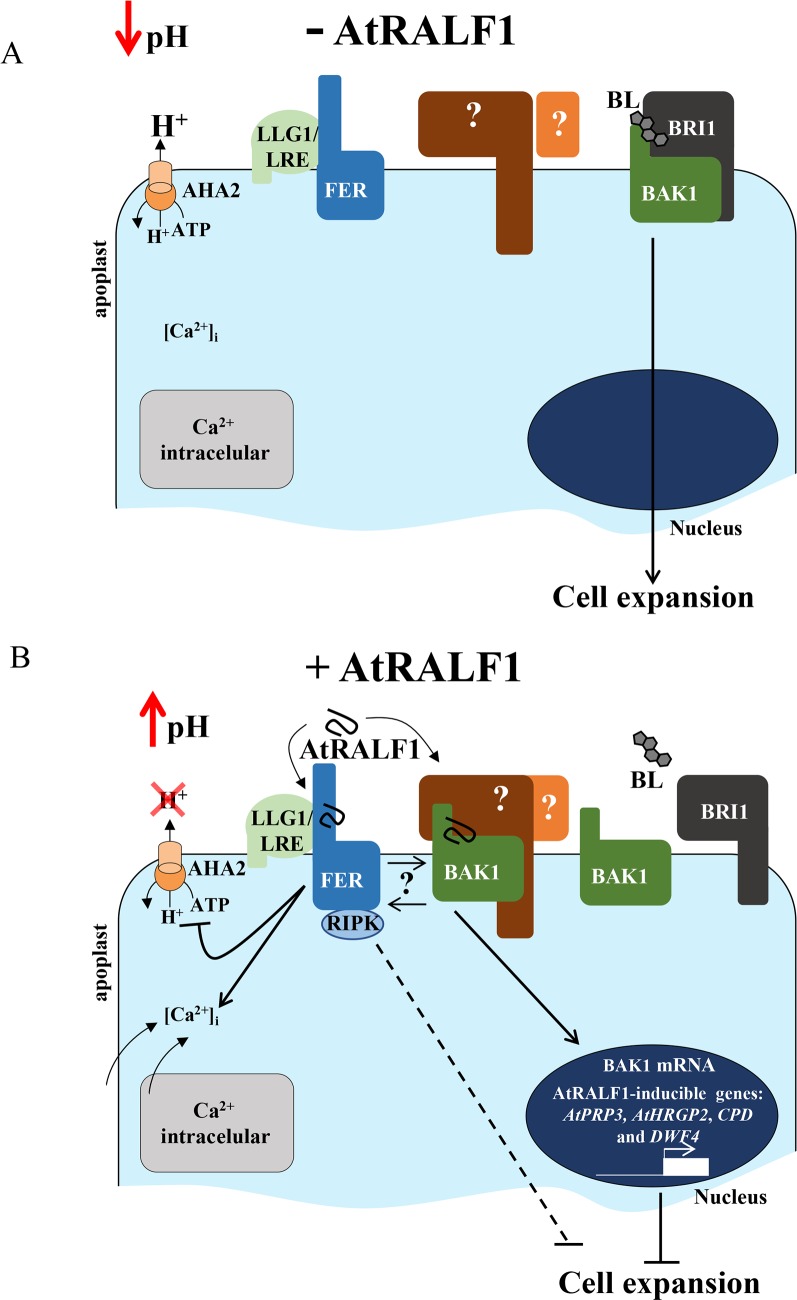
Fig 9. Proposed model for AtRALF1 perception in root cells of Arabidopsis.
(A) In the absence of AtRALF1, the BRI1-BL-BAK1 complex is active in the plasma membrane and the cell expands. The apoplast is acidic as the plasma membrane proton pump AHA2 is functional and FERONIA (FER) is in the cell membrane complexed with LRE-like GPI-AP1 (LLG1)/LORELEI (LRE). (B) In the presence of AtRALF1, the peptide binds FER-LLG1/LRE complex, recruits the receptor-like cytoplasmic kinase RIPK and inactivates AHA2, leading to an increase in apoplastic pH. It is proposed that the resulted apoplastic alkalinization dissociates the BRI1-BL-BAK1 complex, allows AtRALF1 to bind BAK1, disrupts BL signaling, activates AtRALF1-inducible genes and, ultimately, inhibits cell expansion. According to our model, AtRALF1/BAK1-dependent responses are downstream of intracellular Ca2+ mobilization and apoplastic alkalinization. Both FER and BAK1 may interact, and an apoplastic factor, and at least another receptor is expected to play a role in AtRALF1 perception (question marks). The intersecting line indicates incomplete insensitivity to inhibition of cell expansion caused by AtRALF1; solid lines depict direct actions (with bars at the end = inhibition or arrowed = activation). For more details, see the Discussion.