Figure 1.
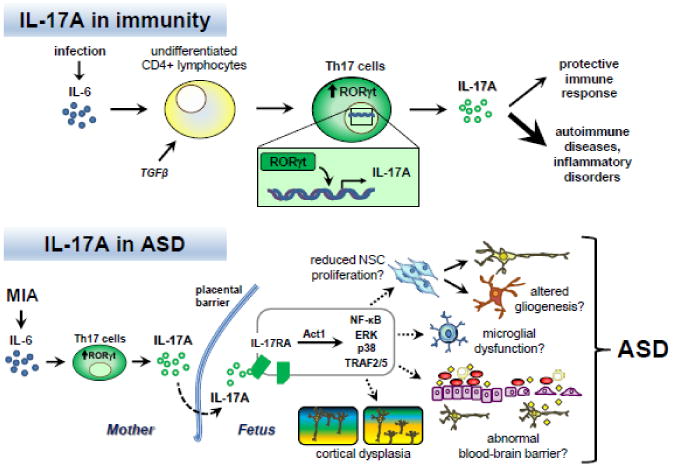
IL-17A and immunity: Infection causes increased expression and secretion of the proinflammatory cytokine interleukin-6 (IL-6). In the presence of the cytokine transforming growth factor β (TGFβ), IL-6 can stimulate the differentiation of naïve CD4+ lymphocytes into T helper 17 (Th17) cells. A key downstream effector of IL-6 involved in Th17 differentiation is the transcription factor retinoic acid receptor-related orphan receptor γt (RORγt). RORγt promotes transcription of the pro-inflammatory cytokine IL-17A. IL-17A signaling plays a protective role in adaptive immunity. Dysregulation of Th17 cells and IL-17A production are associated with autoimmune diseases and inflammatory disorders.
IL-17A and ASD: In the case of maternal immune activation (MIA), IL-6 is induced and stimulates the differentiation of Th17 cells in the mother, leading to increased IL-17A secretion. IL-17A in turn crosses the placental barrier or is induced in the fetus, where it can act on cells expressing the receptor IL-17RA in the developing nervous system. Stimulation of IL-17RA can activate several intracellular signaling pathways mediated by Act1, such as NF-κB, ERK, p38 MAPK, and TRAF2/5. Consequently, various developmental processes can be altered, including neural stem cell (NSC) proliferation, gliogenesis, microglial function, blood-brain barrier activity, and neuronal connectivity. In these ways, inappropriate Th17 cell and IL-17A activity may adversely impact prenatal development, resulting in cortical dysplasia and the manifestation of behaviors associated with ASD in the offspring (Choi et al. 2016).
