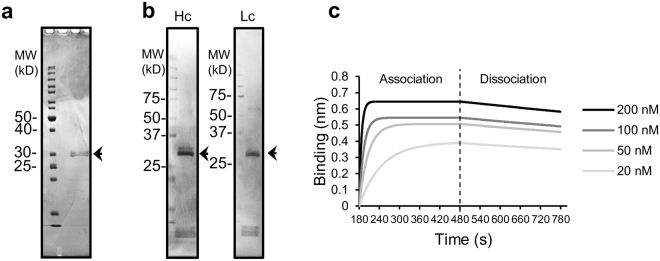
Figure 4.
Analysis of refolded and purified Zipbody mAb produced in E. coli. Refolded and purified anti-V. parahaemolyticus Zipbody clone G22 produced in E. coli SHuffle T7 Express was used. The purified fraction in these analyses was obtained by Ni-affinity chromatography. The left-hand lane in panels a and b indicates molecular size markers. Arrows show the expected size of the Zipbody proteins (which overlap). (a) Coomassie Brilliant Blue staining after SDS-PAGE. One microgram of the purified fraction was loaded. (b) Western blotting detecting Hc and Lc, with anti-HA tag-HRP and anti-His tag-HRP conjugated antibodies, respectively. Loaded protein amount = 0.1 µg. (c) Blitz sensorgram at various Zipbody mAb concentrations against inactivated V. parahaemolyticus cells, corrected with 0 nM Zipbody as the reference.