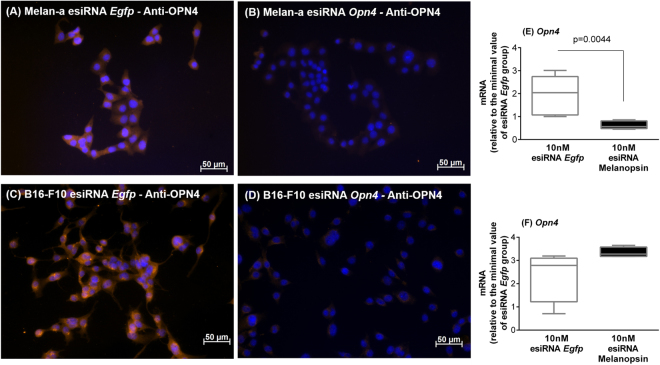
Figure 4.
Representative fields of melanopsin (OPN4) immunostaining in Melan-a (A,B) and B16-F10 (C,D) cells. Cells were kept in DD for 24 hours, on the 2nd day, were transfected with esiRNA against melanopsin or EGFP (both at 10 nM), and 48 h after transfection the cells were immunostained for melanopsin (OPN4). (A and C) esiRNA against mRNA of EGFP transfected cells (control group) and (B and D) esiRNA against mRNA of OPN4 transfected cells. Photo-micrographies were obtained with 200 x magnification in an inverted fluorescence microscope Axiovert 40CFL (Zeiss, Oberkochen, Germany) with a mercury lamp of 50 W, and DAPI (excitation 358 and emission 463 nm) and Cy3 (excitation 549 and emission 562 nm) filters. Melanopsin gene and protein knockdown by endoribonuclease-prepared siRNAs (esiRNA). Gene expression of Opn4 (melanopsin encoding gene) in esiRNA against mRNA of EGFP (control group) or of melanopsin transfected cells. Melan-a (E) or B16-F10 (F) cells were kept during three days in constant dark and temperature (37 °C). At the beginning of the 4th day, Melan-a cells were synchronized by two medium changes, and after further 24 hours they were transfected with esiRNA. B16-F10 cells were transfected at the beginning of the 4th day. In both cases, gene expression was evaluated 48 h after transfection with esiRNA. Boxplots show the median, quartiles, maximum, and minimum expression values of each gene transcript normalized by Rpl 37a and expressed relative to the minimal value of the esiRNA EGFP group kept at 37 °C (N = 5–6). Statistical analysis was performed by Student’s t test.