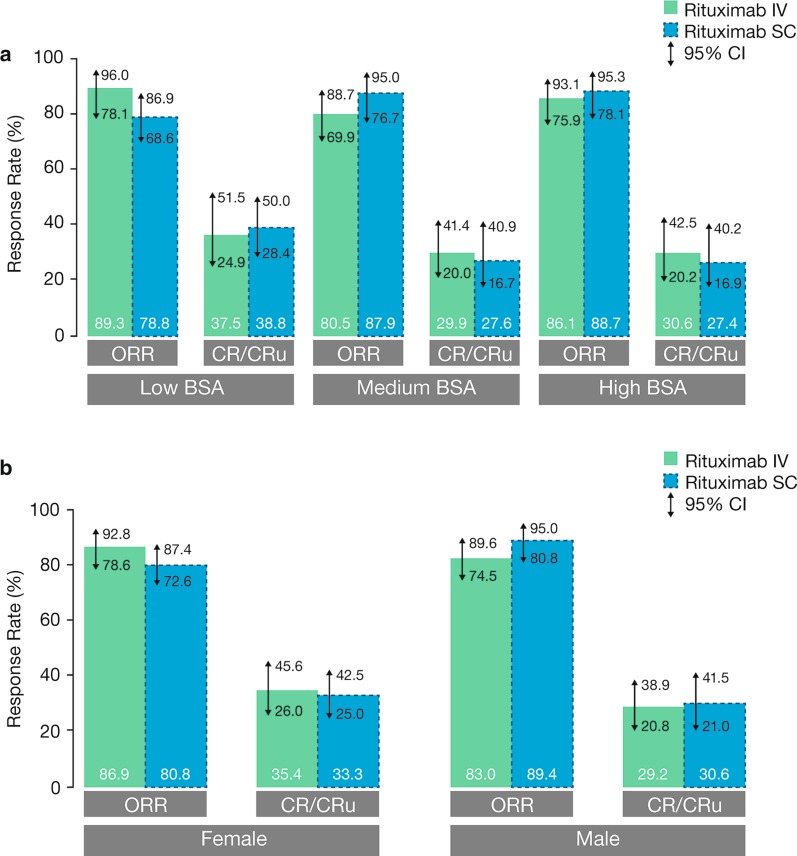
Fig. 3.
OR rate by BSA category (a) [Adapted from Davies et al. 2017] [44] and gender (b) [67] at the end of induction in SABRINA. CI confidence interval, CR complete response, BSA body surface area, IV intravenous, SC subcutaneous
Part A reproduced from The Lancet Haematology, vol. 4, Davies A, Merli F, Mihaljević B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial, e272–82, Copyright (2017), with permission from Elsevier