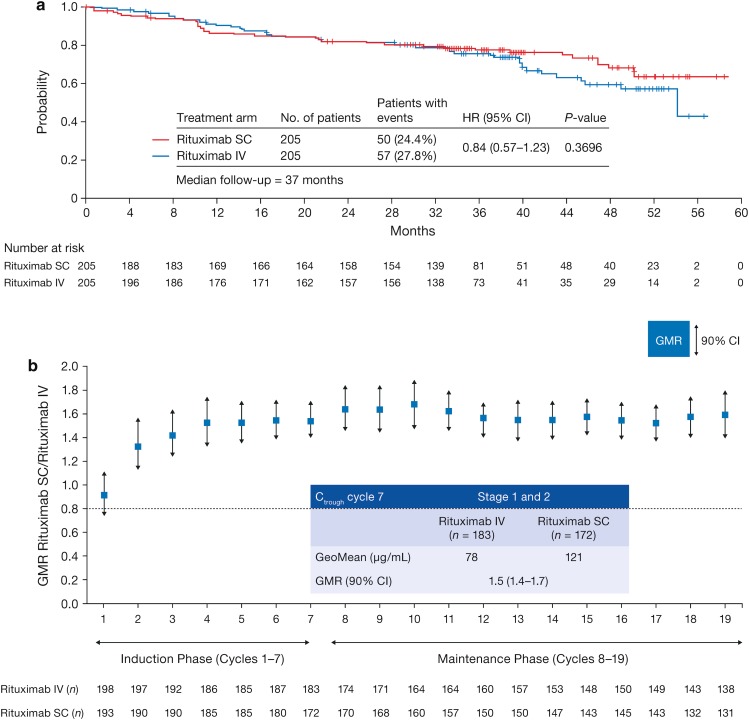
Fig. 4.
Progression-free survival in patients receiving rituximab SC or IV in a the phase III SABRINA study [44]. b Geometric mean ratio non-inferiority of rituximab SC versus IV in SABRINA. CI confidence interval, HR hazard ratio. For time-to-event analyses, a hazard ratio below 1 implies a risk reduction for rituximab SC.
Reproduced from The Lancet Haematology, Vol. 4, Davies A, Merli F, Mihaljević B, et al. Efficacy and safety of subcutaneous rituximab versus intravenous rituximab for first-line treatment of follicular lymphoma (SABRINA): a randomised, open-label, phase 3 trial, e272–82, Copyright (2017), with permission from Elsevier