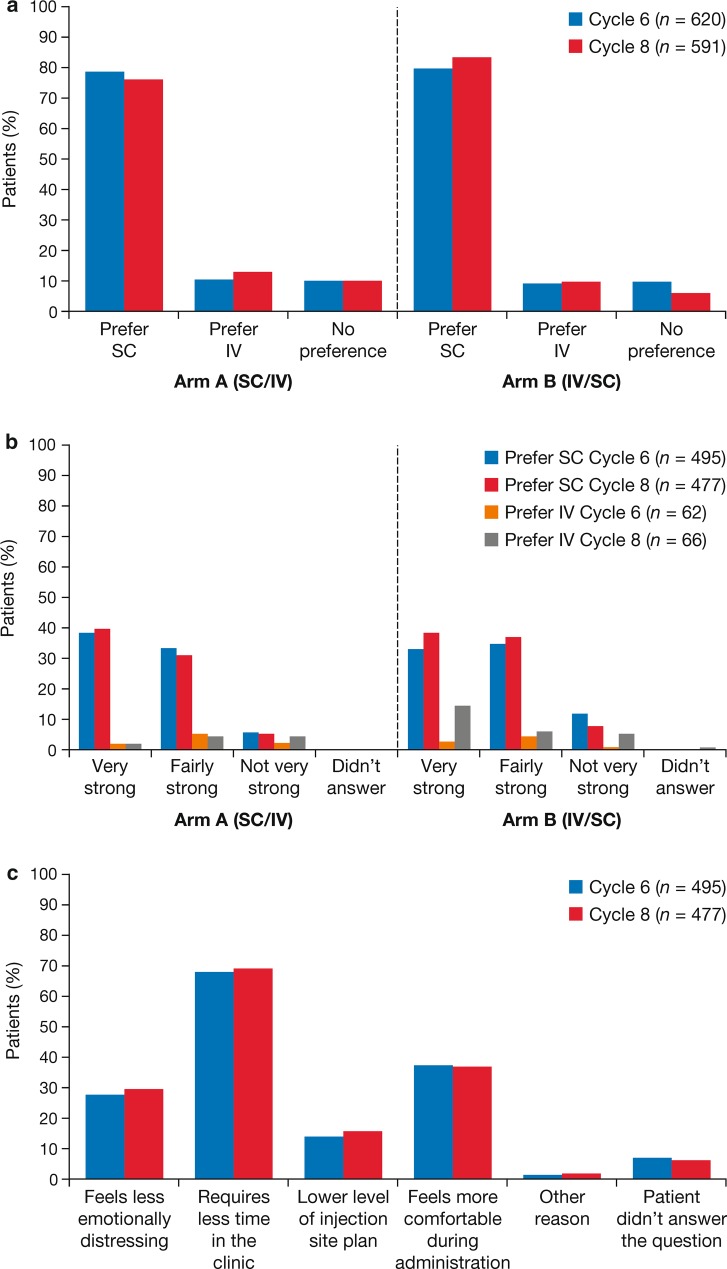
Fig. 5.
Findings from the PrefMab study showing an overall patient preference for the SC or IV route of rituximab administration at cycles 6 and 8; b strength of patient preference for the SC or IV route; c reasons for patient preference in patients preferring SC administration [51]'.
Figure reproduced from Rummel M, Kim TM, Aversa F, et al. Preference for subcutaneous or intravenous administration of rituximab among patients with untreated CD20+ diffuse large B-cell lymphoma or follicular lymphoma: results from a prospective, randomised, open-label, crossover study (PrefMab). Ann Oncol. 2017;28:836–42, by permission of Oxford University Press