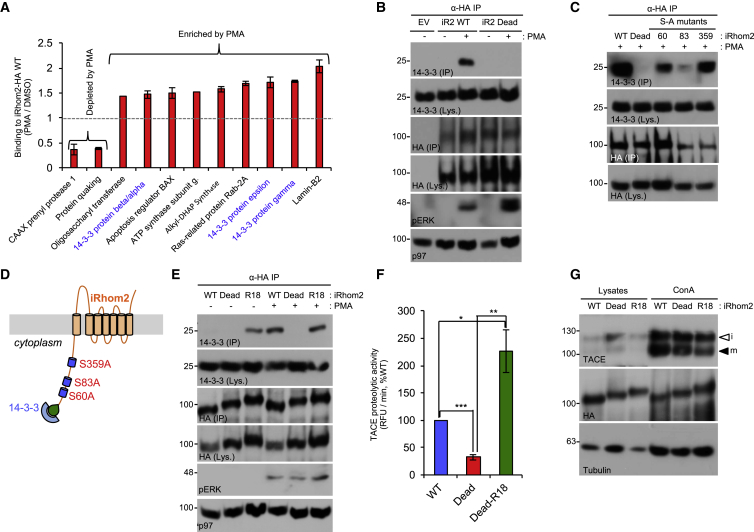
Figure 5.
Phosphorylation-Dependent Recruitment of 14-3-3 to iRhom2 Induces Increased TACE Activity
(A) iRhom2-interacting proteins with altered binding upon PMA stimulation, detected by mass spectrometry, in iRhom2-HA immunoprecipitates; 14-3-3 proteins are highlighted in blue. See Figure S1B for a more comprehensive view of this dataset (n = 2).
(B) Validation of PMA-inducible 14-3-3 binding to iRhom2. HEK293ET cells expressing an empty vector (EV) control, WT, or iRhom2-Dead-HA were stimulated (PMA, 30 min). iRhom2-HA was immunoprecipitated and endogenous 14-3-3 binding detected with a pan-14-3-3 antibody.
(C) S83 is the major phosphorylated residue that recruits 14-3-3 proteins. HEK293ET cells expressing iRhom2-HA serine-to-alanine (S/A) mutants at the three putative 14-3-3 binding sites (S60, S83, and S359) were stimulated with PMA (30 min), iRhom2-HA was immunoprecipitated and endogenous 14-3-3 binding detected.
(D) Schematic of the R18-iRhom2 Dead mutant. The R18 peptide (PHCVPRDLSWLDLEANMCLP) was fused to the N terminus of iRhom2 Dead-HA mutant, to confer phosphorylation independent binding of 14-3-3 proteins.
(E) The R18-iRhom2 Dead-HA mutant binds constitutively to endogenous 14-3-3 proteins. HEK293ET cells expressing the indicated plasmids were stimulated (PMA, 30 min).
(F) 14-3-3 binding induces TACE cell surface proteolytic activity. HEK293ET cells overexpressing the indicated plasmids were left untreated. TACE proteolytic activity on the cell surface was determined by measuring its ability to cleave a fluorogenic substrate. RFU, relative fluorescent units. Student’s t test.
(G) TACE maturation is not affected by 14-3-3 binding. Lysates from iRhom1/2 DKO MEFs stably expressing the indicated plasmids were ConA enriched and immunoblotted for TACE.
Data are presented as mean ± SEM. See also Figure S1.