Figure 2.
Activation of IIS Results in FKH Phosphorylation, and FKH Interacts with AKT and TOR In Vitro
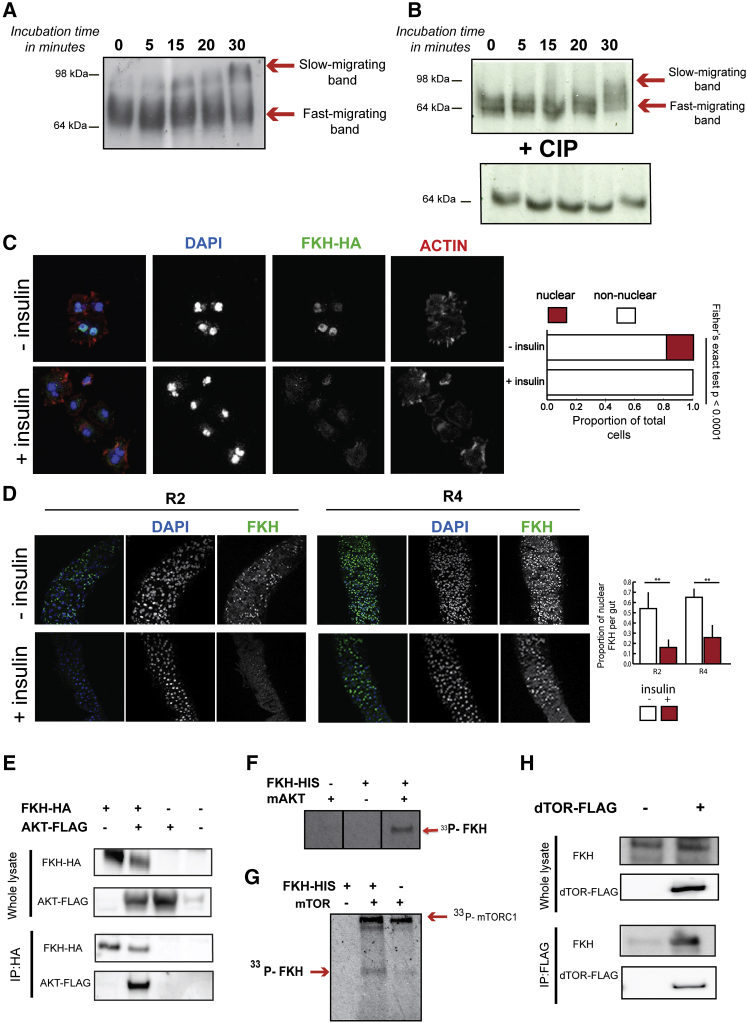
(A) S2 cells were transfected with HA-tagged FKH construct and stimulated with insulin for 5, 15, 20, and 30 min. A band above 64 kDa was detected in extracts pre-insulin stimulation. A slower migrating band at ∼98 kDa was present in extracts isolated post-insulin stimulation.
(B) Following phosphatase treatment of the same extracts, a single band corresponding to non-phosphorylated FKH-HA was present (lower panel).
(C) S2 cells were visualized for FKH-HA protein (green), Actin (red), and nuclei stained by DAPI (blue) pre-and post-insulin treatment for 20 min. Quantification of nuclear FKH revealed a significant difference between two conditions (Fisher’s exact test, p < 0.0001).
(D) Ex vivo insulin-stimulated guts were stained for endogenous FKH (green) and DAPI-stained nuclei (blue) and in two different sections of midgut: R2 (left panel) and R4 (right panel). Initially strong nuclear FKH staining was markedly decreased in both R2 (t test, p = 0.045; n = 4) and R4 (t test, p = 0.0014; n = 4) upon insulin stimulation. Error bars represent SD.
(E) S2 cells were transiently transfected with the indicated cDNAs in expression vectors. Anti-HA immunoprecipitates were prepared, and cell lysates were analyzed by immunoblotting with either anti-HA or anti-FLAG antibodies.
(F and G) Recombinant FKH-HIS was incubated in an in vitro kinase reaction with active recombinant (F) Akt1 or (G) mTORC1 radiolabelled [γ-33P]ATP. Reactions lacking either the substrate or the kinase served as negative controls. Proteins were separated by SDS-PAGE and phosphorylated proteins were visualized by autoradiography. In reactions with mTORC1, the incorporation of [γ-33P]ATP was also observed at a higher molecular weight (∼160 kDa), which probably reflected the previously reported phosphorylation of raptor by mTOR (Wang et al., 2009).
(H) S2 cells were transiently transfected with the dTOR-FLAG vector. Anti-FLAG immunoprecipitates were prepared, and cell lysates were analyzed by immunoblotting with either anti-FKH or anti-FLAG antibodies.