Scheme 4.
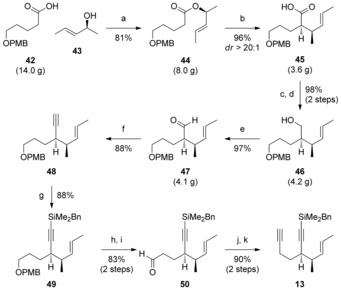
Reagents and conditions: a) EDC⋅HCl, NEt3, DMAP; b) LiHMDS, NEt3, PhMe, −78 °C→RT; then 5 % NaOH; then conc. HCl; c) TMSCHN2, MeOH/PhMe, 0 °C→RT; d) DIBALH, CH2Cl2, −78 °C→RT; e) DMP, NaHCO3, CH2Cl2; f) NaHMDS, [Ph3PCH2I]I, THF, −78 °C→RT; NaHMDS, −78 °C→RT; g) LiHMDS, THF, −78 °C; BnMe2SiCl, −78 °C to RT, 88 %; h) DDQ, CH2Cl2; i) DMP, NaHCO3, CH2Cl2; j) CBr4, PPh3, CH2Cl2, 0 °C; then 50, NEt3, −30 °C→0 °C; k) nBuLi, THF, −78 °C→RT. DDQ=2,3‐Dichloro‐5,6‐dicyano‐1,4‐benzoquinone; DMP=Dess–Martin periodinane; EDC=1‐ethyl‐3‐(3‐dimethylaminopropyl) carbodiimide.
