Scheme 2.
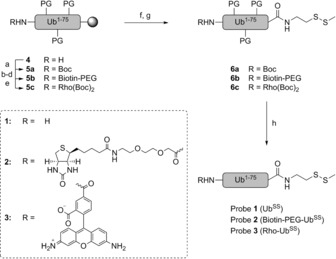
Synthesis of disulfide probes 1 (UbSS), 2 (biotin–PEG–UbSS), and 3 (Rho–UbSS). Ubiquitin lacking the C‐terminal glycine (Ub1–75) was synthesized on resin (4). The N‐terminus was modified under various conditions (a–e). a) Boc2O, DiPEA, NMP; b) PyBOP, DiPEA, (8‐Fmoc‐amino)‐3,6‐dioxaoctanoic acid, NMP; c) piperidine, NMP: d) biotin, HBTU, HOBt, DiPEA, NMP; e) PyBOP, DiPEA, N,N′‐Boc‐protected 5‐carboxyrhodamine 110 (Rho),6 NMP. After cleavage from the resin (f), the C‐terminus was modified with an S−SMe group (g). Global deprotection yielded the final probes (h). f) HFIP, DCM; g) PyBOP, Et3N, 2‐(methyldisulfanyl)ethan‐1‐amine, DCM; h) TFA/H2O/iPr3SiH/PhOH. See the Supporting Information for details. DCM=dichloromethane, DiPEA=N,N‐diisopropylethylamine, HBTU=O‐(benzotriazol‐1‐yl)‐N,N,N′,N′‐tetramethyluronium hexafluorophosphate, HFIP=hexafluoroisopropanol, HOBt=1‐hydroxybenzotriazole, NMP=N‐methyl‐2‐pyrrolidone, PyBOP=benzotriazol‐1‐yl‐oxytripyrrolidinophosphonium hexafluorophosphate, TFA=trifluoroacetic acid.
