Table 1. Probing the importance of boron positioning and substitution on oxime condensations.
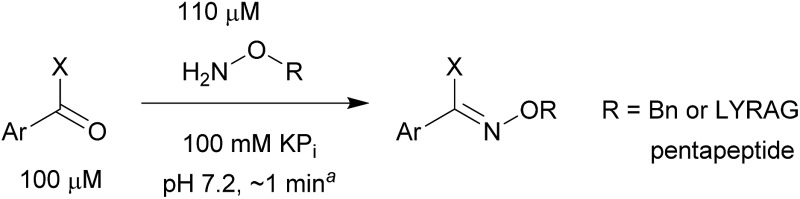
| |||||
| Entry | Ar | X | Product | Conc (µM) | Conv b (%) |
| 1 c | Ph | H |
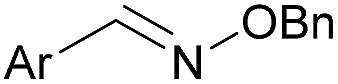
|
100 | <5 |
| 2 |
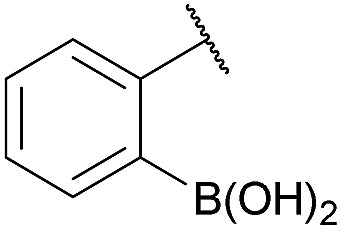
|
H |

|
100 | >98 |
| 3 |
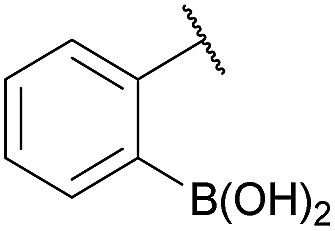
|
H |
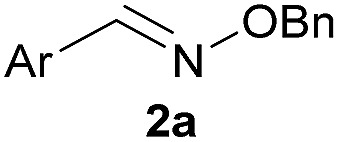
|
10 | >98 |
| 4 |
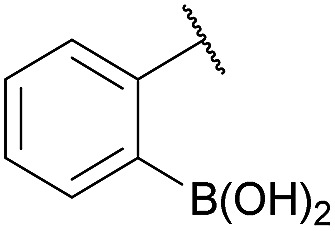
|
H |
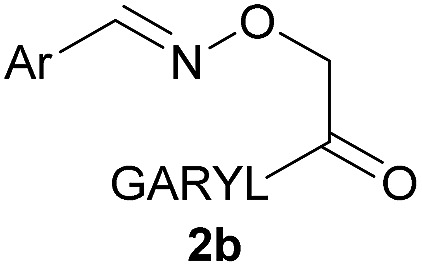
|
100 | >98 |
| 5 c |
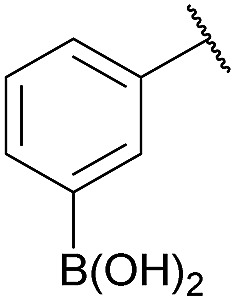
|
H |
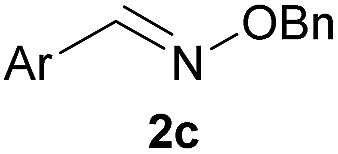
|
100 | <5 |
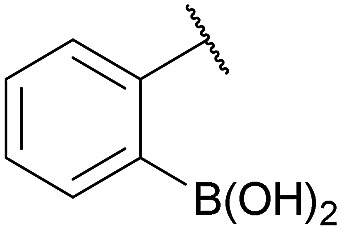
| 6 c |
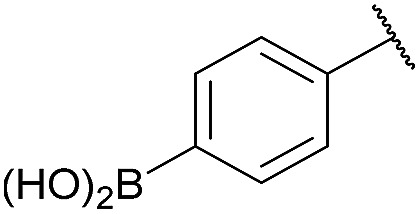
|
H |
|
100 | <5 |
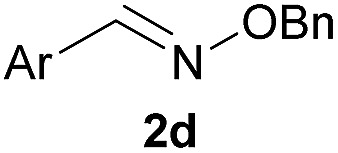
| 7 |
|
Me |
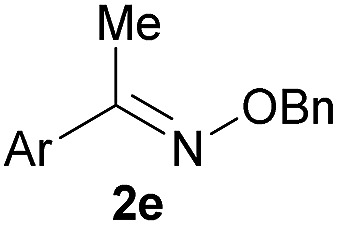
|
100 | 94 |
| 8 |
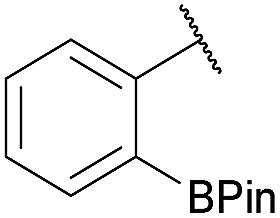
|
H |
|
100 | >98 d |
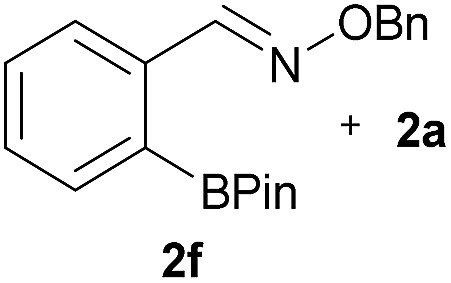
| 9 |
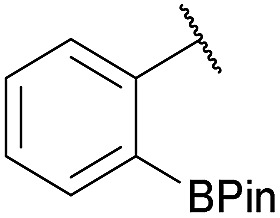
|
Me |
|
100 | >98 |
aTime is approximate since samples are injected directly after mixing.
bDetermined by reverse phase HPLC analysis under neutral conditions.
cInjections at 90 minutes still show <5% conversion.
dAt the first injection approximately 10% of the pinacol ester oxime is observed, but only the hydrolysed product is detected at 90 minutes. KPi = potassium phosphate buffer.
