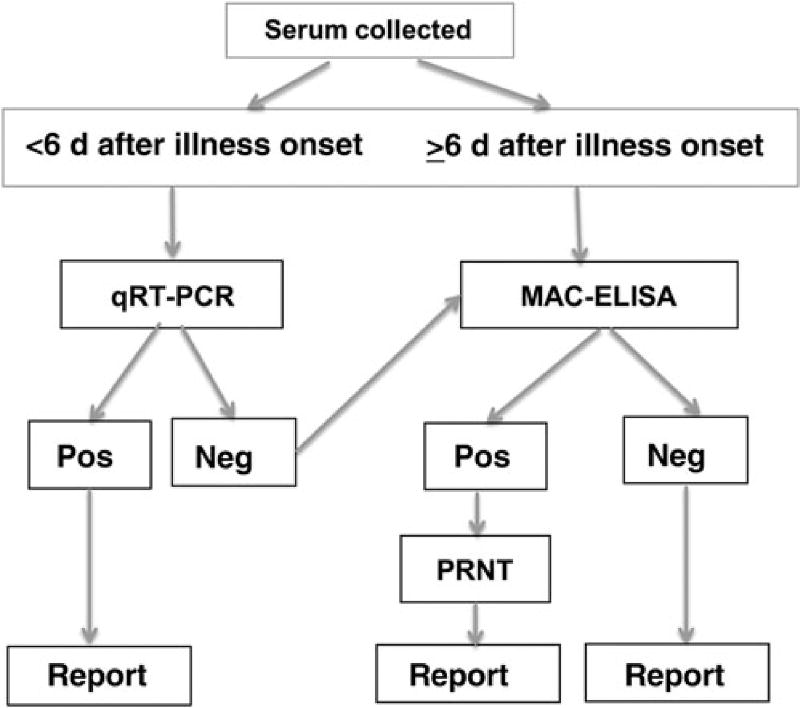
Figure 2.
The Centers for Disease Control and Prevention diagnostic testing algorithm for detection of chikungunya virus (CHIKV) infection. Serum is collected from patients meeting the clinical case definition of fever and arthralgia who have returned from a region where CHIKV is endemic or CHIKV infection is epidemic. All samples with positive and equivocal real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) results are repeated with a second set of primer/ probes for confirmation. Specimens with positive results of tests using both sets of primers and probes are considered to be confirmed CHIKV-positive specimens. Samples with positive or equivocal immunoglobulin M capture enzyme-linked immunosorbent assay (MAC-ELISA) results are confirmed to be positive if plaque reduction neutralizing testing (PRNT) yields positive results. Abbreviations: Neg, negative result; Pos, positive result.