Figure 2. Schematic representation of post-translational prenylation of G-proteins.
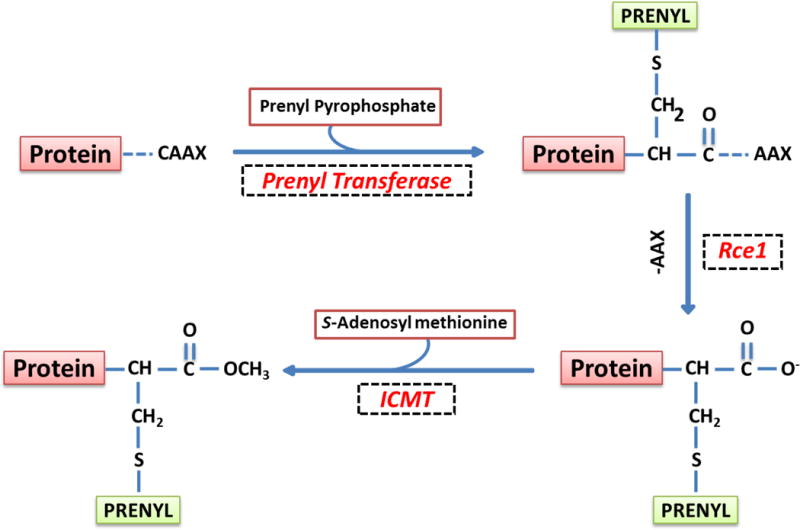
The first of the four-step modification is incorporation of either a farnesyl or a geranylgeranyl derivative of MVA into the carboxy terminal cysteine [CAAX motif] via a thioether linkage. Farnesylation and geranylgeranylation steps are catalyzed by the FTase and the GGTase, respectively. Collectively, they are referred to as prenyl transferase. Following prenylation, the three amino acids after the prenylated cysteine [AAX] are removed by a protease [Rce1] of microsomal origin, thereby exposing the carboxylate anion. This site is then methylated by carboxyl methyl transferase [ICMT], which transfers a methyl group onto the carboxylate group using S-adenosyl methionine [SAM] as the methyl donor. The CML of G-proteins increases their hydrophobicity and translocation to the membrane fraction. In addition to these, certain G-proteins [H-Ras and Rac1] have been shown to undergo palmitoylation at a cysteine residue, which is upstream to the prenylated cysteine. It is thought that palmitoylation provides a “firm” anchoring for the modified protein into the cell membrane for optimal interaction with its respective effector proteins. [Reproduced with permission from Elsevier].19
