Figure 1.
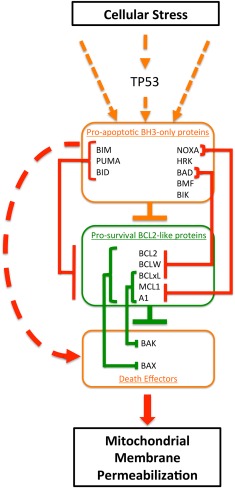
Overview of the regulation of the intrinsic pathway to apoptosis by B‐cell‐lymphoma‐2 (BCL2) family members. Within the cytoplasm of normal cells, apoptosis is regulated by highly specific interactions between three subfamilies of the BCL2 protein family. The BCL2 homology (BH)3‐only proteins integrate a multitude of stress‐induced signals, and apoptosis is unleashed when the net BH3‐only pro‐apoptotic activity exceeds the activity of the prosurvival proteins, most prominent of which is BCL2. In healthy cells, BCL2 and structurally and functionally related proteins, such as MCL1 or BCLxL, bind and repress the activity of the third subfamily of BCL2‐like proteins, the death effectors (mediators) BAX and BAK. When sufficient stress signals are applied, prosurvival proteins are displaced from BAX/BAK by interaction with BH3‐only proteins, allowing BAX and BAK to oligomerize on the outer membrane of mitochondria, triggering its permeabilization, depolarization, cytochrome C release, caspase activation, and cell death, morphologically recognizable as apoptosis. Stresses related to DNA damage from chemotherapy and from oncogenic signaling typically induce BH3‐only protein activity via the TP53 pathway. Interactions between BH3‐only proteins and prosurvival proteins can be specific (e.g., BAD only binds BCL2, BCLxL, and BCLW with high affinity; and BCL2 preferentially binds and inhibits BAX), or more promiscuous (e.g., BIM will bind and inhibit all prosurvival proteins, and MCL1 will bind and inhibit both BAX and BAK).7 Orange boxes and orange lines represent apoptosis inducing proteins and actions. The red lines indicate the pro‐apoptotic action of BH3‐only proteins. Green boxes and lines represent survival promoting proteins and their actions. Lines with arrows indicate signals that enhance activity, whereas lines headed with bars indicate repressive actions.
