Figure 2.
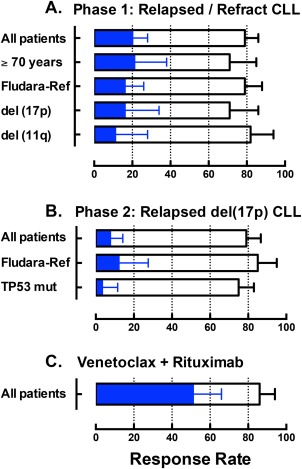
The graph summarizes previously published or presented overall response and complete remission rates for patients with relapsed or refractory chronic lymphocytic leukemia (CLL) treated with venetoclax on early phase clinical trials. Open bars represent the overall response rate and the blue bars indicate the complete remission rate, as assessed against International Workshop on CLL 2008 criteria. The error bars indicate the upper 95% confidence interval for the response rates. (a) Pooled data across all dose cohorts for 116 patients entering the first‐in‐human phase I study.45 (b) Independent review committee‐assessed data for the 107 patients with del(17p) CLL entering the phase II trial at the now‐approved 400 mg/day dose.47 (c) Pooled data across all dose cohorts for 49 patients treated with the combination of venetoclax and rituximab (6 doses only) on the phase Ib trial.49, 65 Fludara‐Ref, refractory to previous fludarabine‐containing therapy.
