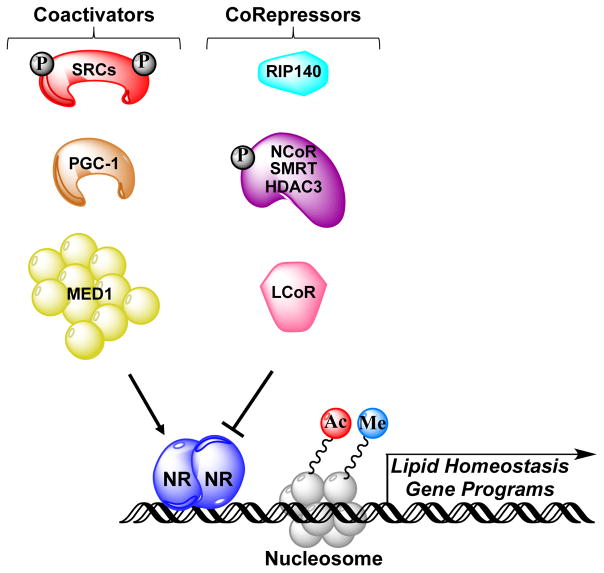
Figure 4. Potential genomic mechanism of EDC action.
Once an endocrine-disrupting chemical (EDC) enters the liver, it is bound by specific nuclear hormone receptors (NRs). This action can either positively or negatively affect transcription of lipid homeostasis genes via specific EDC–NR complexes that recruit coactivators or corepressors to target genes. Key coactivators that modulate fatty liver progression include steroid receptor coactivators (SRCs), peroxisome proliferator-activated receptor γ coactivator 1α (PGC1α) and mediator of RNA polymerase II transcription subunit 1 (MED1), whereas nuclear receptor corepressor (NCoR)–silencing mediator of retinoic acid and thyroid hormone receptor (SMRT)–histone deacetylase 3 (HDAC3) complexes, receptor-interacting protein 140 (RIP140) and ligand-dependent corepressor (LCOR) act as corepressors. Coactivator complexes induce histone modifications associated with active gene transcription, such as acetylation (Ac) and methylation (Me), whereas corepressors generally utilize associated histone deacetylases or demethylases to remove these marks. SRCs and NCoR are subject to regulatory phosphorylation (P) events. Engagement of coregulators by EDC-bound NRs results in modulation of lipid homeostasis gene cassettes and/or reprogramming of the epigenome, which ultimately promotes NAFLD: for example, via enhanced lipogenesis gene expression and/or inhibition of free fatty acid-oxidation gene expression.