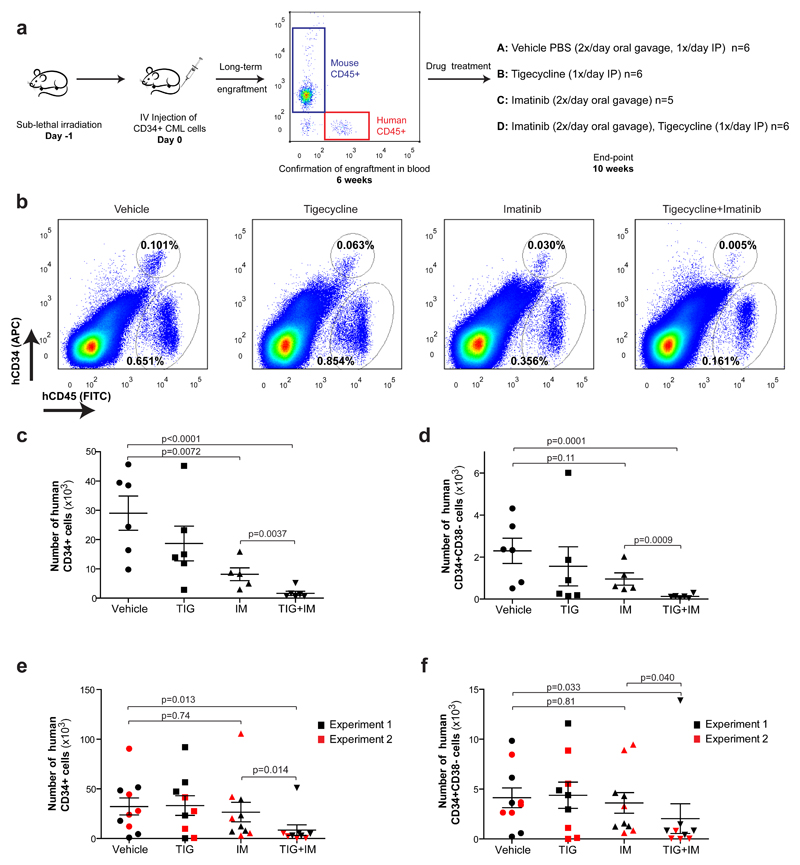
Figure 4. Inhibition of oxidative metabolism eliminates xenotransplanted human CML LSCs.
(a) Diagram of experimental design. The pre-treatment engraftment levels of CML cells in mice were assessed by monitoring the percentage of human CD45+ circulating leukocytes using flow cytometry. (b) Representative analyses of human CD45 and CD34 expression in murine bone marrow was used to assess engrafted CML cells following the indicated treatment. (c) Number of human CD34+ and (d) human CD34+CD38- CML cells remaining in the bone marrow following in vivo drug treatment. (e) Number of human CD34+ and (f) human CD34+CD38- CML cells remaining in the bone marrow following 2 (experiment 1) or 3 (experiment 2) weeks of drug discontinuation. n≥5 mice per treatment arm. TIG, tigecycline; IM, imatinib. P-values were calculated by unpaired Student’s t-test on logarithmically transformed variables to meet the assumption of normality.