Figure 2.
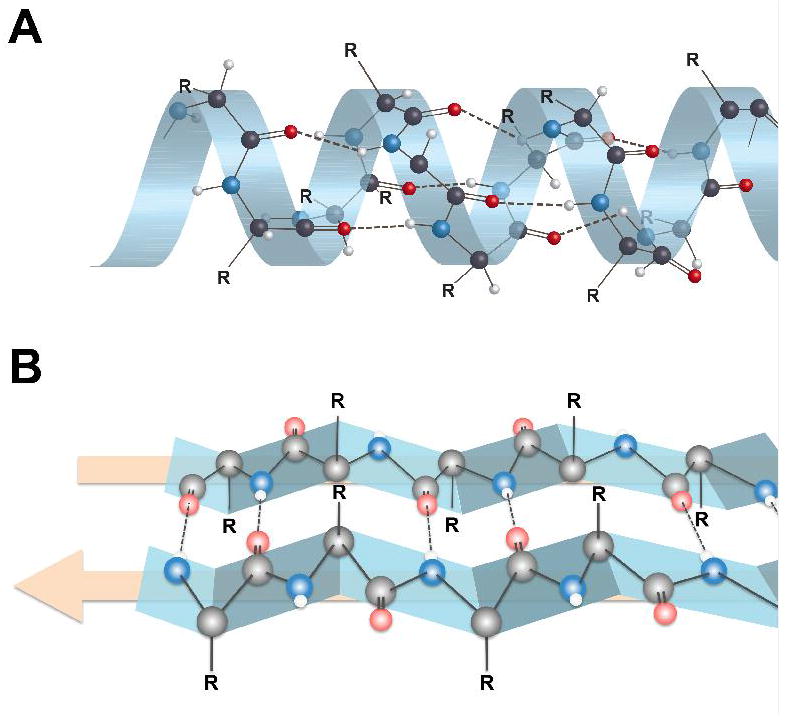
Secondary structures of proteins showing alpha-helix (A) and beta-pleated sheet (B) configurations. Hydrogen bonding between an amide nitrogen (blue) and an amide oxygen (red) in beta-pleated sheet structures takes place at the interfaces between two protein strands (edge-on-edge interactions), which is very different than the intramolecular hydrogen bonding of alpha-helix proteins. Note that the R groups on the α-carbons are either above the beta-sheet or below the beta-sheet. This presents sites for potentially different binding interactions with ligands on the two sides of the beta-sheet. Figure 4 displays the R group orientations from a different perspective.
