FIGURE 2.
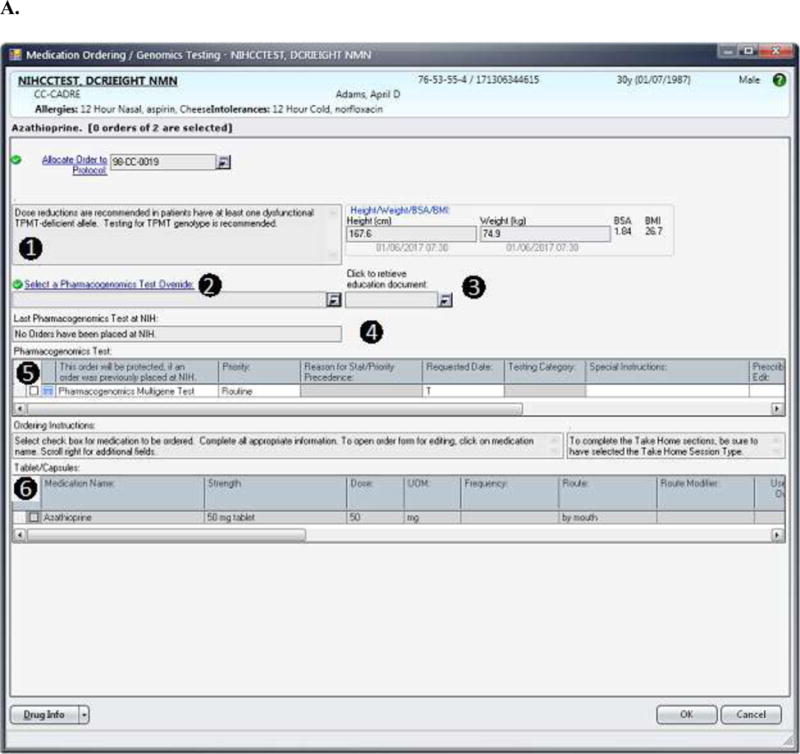
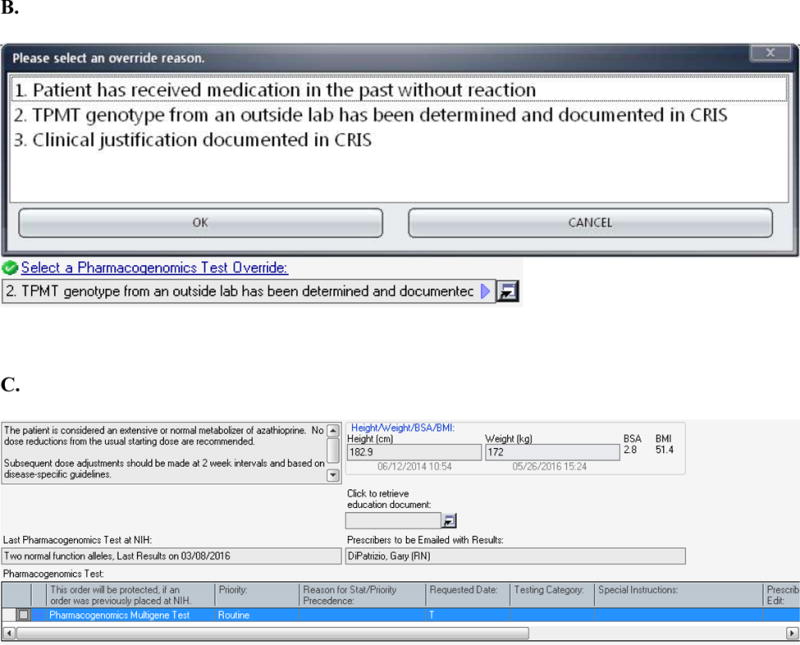
(A) The design for the order set form for drugs included in the pharmacogenomics DMET program is shown. In this case, the information displayed to the prescriber is based on the case where the Pharmacogenomics Multigene Test has not yet been ordered. The displayed messages and order actions are determined by the control table. The standard design for the order set form includes (1) a message box where the clinical information message is displayed, and (2) a message box where over-ride reasons are displayed (the ‘over-ride reason number’ field becomes a required entry if an over-ride reason is allowed), (3) a link to the Education Document to provide to the patient, (4) a message box where pharmacogenomics test information and when appropriate result information is displayed, (5) a grid where the Pharmacogenomics Multigene Test can be ordered or are automatically preselected depending on the case, and (6) a grid where medications can be ordered through the Clinical Research Information System. (B) The top image shows the modal window showing the override options. The bottom image shows how override reason 2 will display when selected. (C) The image shows information related to the actual result of the Pharmacogenomics Multigene Test. The MLMs uses a control table to process clinical rules for medication orders. The control table allows the management of the process through logic defined in the MLM and also allows the user to add new medications or add or modify rules for test results as needed to refine the logic. Each medication within the pharmacogenomics DMET program will have entries in the control table as seen in Table 1. This report is a sample and does not refer to an actual patient.
