Fig 3. Molecular characterization of Rhesus family C glycoprotein (Rhcg) from the gills of Protopterus annectens.
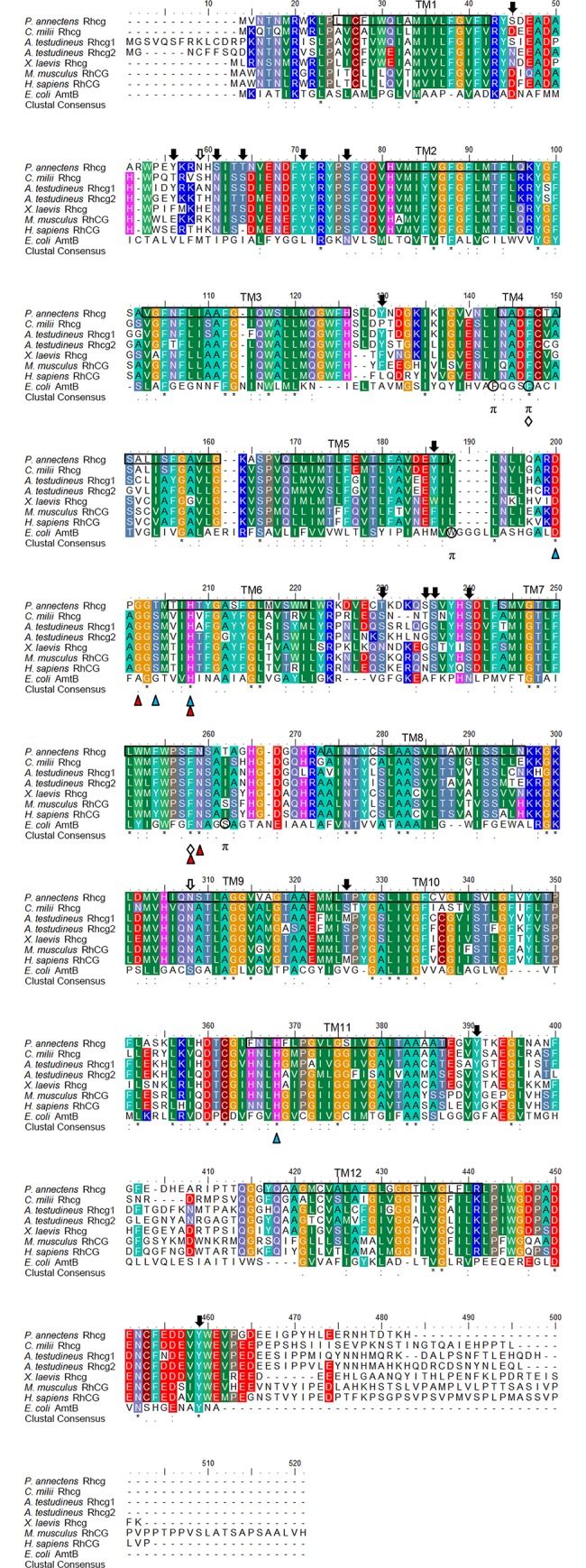
A multiple amino acid alignment of Rhcg from Protopterus annectens with Callorhinchus milii Rhcg (AFO96383.1), Anabas testudineus Rhcg1 (AIC81183.1) and Rhcg2 (AIC81184.1), Xenopus laevis Rhcg (NP_001088553.1), Mus musculus RhCG (AAF19373.1), Homo sapiens RhCG (AAF19372.1) and Escherichia coli ammonia transporter (AmtB; NP_414985.1). Identical amino acid residues are indicated by asterisks, strongly similar amino acids are indicated by colons and weakly similar amino acids are indicated by periods. Residues involved in NH4+ binding and deprotonation of NH4+ for NH3 conduction are indicated by red and blue triangles, respectively. The phenylalanine gate is indicated by open diamonds. The π cation binding sites of E. coli AmtB are indicated by “π”. Potential N-glycosylation and phosphorylation sites are indicated by open and shaded arrows, respectively. The predicted transmembrane domains (TM1‒12) of Rhcg of P. annectens are indicated by open boxes and were predicted using MEMSATS and MEMSAT-SVA provided by PSIPRED protein structure prediction server.
