Figure 1.
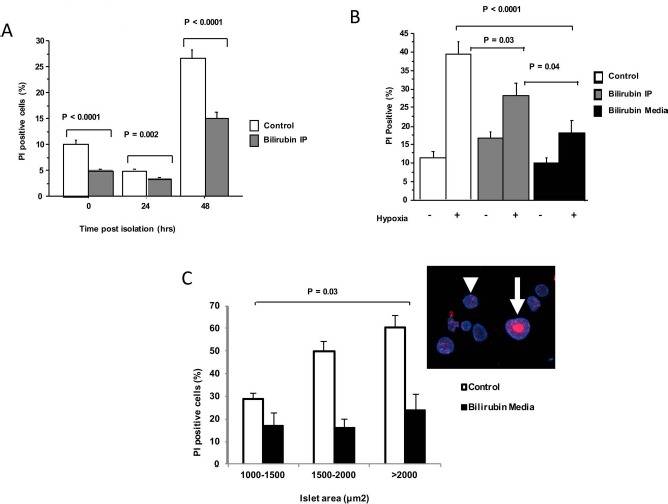
Bilirubin decreases islet cell death in models of transplant-associated stress. AJ mouse islets (100–150 per treatment group) were isolated and maintained in tissue culture (37°C). Dual staining with Hoechst and propidium iodide (PI) was performed, and cell death was estimated based on % PI+ staining. Graphs represent pooled data from three independent experiments in each model. (A) Islets were maintained in culture for 48 h after pancreas digestion and gradient isolation to illustrate the effects of isolation stress. Media were unchanged to cause nutrient deprivation. Progressive cell death occurred, peaking at 48 h after recovery. Bilirubin (10 mg/kg) administered intraperitoneally to the islet donor 1 h before islet harvest (Bilirubin IP) caused significant decreases in cell death. (B) Hypoxic stress was induced by subjecting the islets to 1% O2 for 3 h. Viability was assessed after 24 h of reoxygenation. Hypoxic stress caused marked cell death in the control group. Cell death was significantly decreased by bilirubin IP treatment of the donor prior to islet recovery, but cell death was most effectively prevented by the addition of bilirubin (20 μM/L) to the cell culture media (Bilirubin Media) 1 h before hypoxia. (C) Islets were divided into three size groups based on the measured area of each islet. Percent cell death was greater in large islets exposed to hypoxia, and there was a significant interaction detected between bilirubin treatment and islet size, with bilirubin offering the greatest protection against necrosis of large islets (p = 0.03). A representative image of control islets (inset) shows the large area of central necrosis (arrow) caused by hypoxic injury, while smaller islets were preserved (arrowhead).
